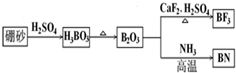
��Ŀ����
7���±����й����ӷ���ʽ�����ۺ������ǣ�������| ѡ�� | ��ѧ��Ӧ�����ӷ���ʽ | ���� |
| A | AlCl3��Һ�м��������ˮ�� Al3++3NH3•H2O�TAl��OH��3��+3NH4+ | ������Ԫ�صIJ���Ӧ����AlO2- |
| B | ��������ͨ���廯������Һ�У� 3Cl2+2Fe2++4Br-�T6Cl-+2Fe3++2Br2 | ��ȷ |
| C | �ð�ˮ���չ����������� 2NH3•H2O+SO2�T2NH4++SO32-ʮH2O | ��ȷ |
| D | �Ȼ������ˮ�� NH4++2H2O�TH3O++NH3•H2O | �����Ȼ���ܽ���ˮ�������仯������д���ӷ���ʽ |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A���Ȼ�����Һ�м�������İ�ˮ���������������ڰ�ˮ�������ӷ���ʽ��ȷ�����۴���
B�������������������Ӻ������Ӷ���������
C�����������������Ӧ������������泥�
D��笠����ӵ�ˮ������ӷ���ʽӦ���ÿ���ţ�
��� �⣺A���������������ڰ�ˮ�����߷�Ӧ�����ӷ���ʽΪ��Al3++3NH3•H2O�TAl��OH��3��+3NH4+���������ӷ���ʽ��ȷ�����۲���������A����
B����������ͨ���廯������Һ�У��������Ӻ������Ӷ���ȫ����������Ӧ�����ӷ���ʽΪ��3Cl2+2Fe2++4Br-�T6Cl-+2Fe3++2Br2���������ۺ�������B��ȷ��
C���ð�ˮ���չ�����������Ӧ���ɵ�����������泥���ȷ�����ӷ���ʽΪ��NH3•H2O+SO2�T2NH4++HSO3-���������ӷ���ʽ�������۴���C����
D���Ȼ������ˮ��笠����ӷ���ˮ�⣬ˮ��Ϊ���淴Ӧ����ȷ�����ӷ���ʽΪ��NH4++2H2O?H3O++NH3•H2O���������۲���������D����
��ѡB��
���� ���⿼���˳�����ѧ�������д�����ۣ���Ŀ�Ѷ��еȣ�ע���������ӷ���ʽ��дԭ���ε�ˮ��ԭ������Ӧ�÷����������йؼ�ץס���Ҫ�Ի�ѧ��������ۺ������ǡ�����ֻ�ж������Ƿ�����������ж����ӷ���ʽ��д�Ƿ���ȷ��Ϊ�״��㣮

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | FeCl3��Һ��KSCN��Һ��� | B�� | п����ϡ������ | ||
| C�� | ͭƬ�����Ȼ�����Һ�� | D�� | ����ͭ��Һ������������Һ��� |
| A�� | 1��2-�������� | B�� | 2-�һ�-3-��ϩ | ||
| C�� | 3��4-����-4-���� | D�� | 3-��-2-�촼 |
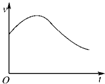
 C��g��+3D��g������H��0���ֽ�2 mol A��2 mol B�������ΪV�ļ���������2 mol C��6 mol D������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V����ͼ1����
C��g��+3D��g������H��0���ֽ�2 mol A��2 mol B�������ΪV�ļ���������2 mol C��6 mol D������������ʹ�������ڷ�Ӧ��ʼǰ�����Ϊ2V����ͼ1����
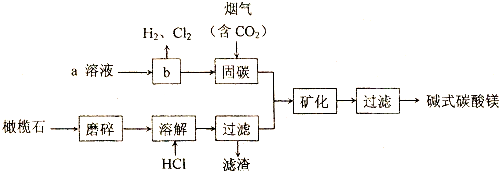

 ����غ����������Ʒ���������ԭ��Ӧ����Cl��-1�ۣ���S��+6�ۣ���������ͼ��ʾ����֪�����Ӧ����������Һ��c��H+��������ӿ죮
����غ����������Ʒ���������ԭ��Ӧ����Cl��-1�ۣ���S��+6�ۣ���������ͼ��ʾ����֪�����Ӧ����������Һ��c��H+��������ӿ죮 ��
��