��Ŀ����
12��ˮ������֮Դ���������ǵ�����������أ��ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮҲ��һ�ֳ��õ��Լ�����1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ͼΪ
 ��
����2��д����H2O���ӵ�����ԭ�ӵ��ӻ���ʽsp3�����������幹��ΪV�Σ�
��3��ˮ�������ض����������õ�һ��H+���γ�ˮ�������ӣ�H3O+�������ж��������̵�������������BCD
A����ԭ�ӵ��ӻ����ͷ����˸ı� B�����еļ��Ƿ����˸ı�
C��������״�����˸ı� D�����Ļ�ѧ���ʷ����˸ı�
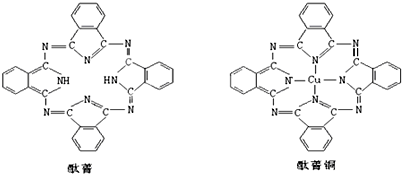
��4������ɫ����ˮCuSO4�ܽ���ˮ�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӣ����������ӵ�����������Cu2+��������H2O����λ����4�������ɫ��Һ�м�������������Һ������Ϊ������ɫ��״�������������백ˮ����������ܽ��γ�����ɫ��Һ����ԭ����Cu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-���÷���ʽ�����ӷ���ʽ��ʾ����
���� ��1������ԭ�Ӻ�������Ų�ԭ�������ȵ���ռ��1�������������������ͬ�ǣ�������ͣ����ݴ�ԭ��Oԭ�Ӻ�����8�����ӣ��ݴ˷������
��2�����ݼ۲���ӶԻ�������ȷ�����ռ乹�ͼ�ԭ���ӻ���ʽ��
��3��H2O��H3O+��Oԭ�Ӷ�����sp3�ӻ�����H2O��H3O+�Ŀռ乹�ͷֱ���V�κ������Σ�
��4������ͭ����ˮ��ͭ������ˮ�����˳���ɫ���������[Cu��H2O��4]2+�������������[Cu��H2O��4]2+��������λ�壺�ṩ�µ��ӶԵķ��ӻ����ӣ���λ��������ԭ����Χ����λԭ�Ӹ���������ͭ���������Ʒ�Ӧ����������ͭ��ɫ��״���������백ˮ�����İ���ͭ������ӣ�
��� �⣺��1�����ڼ�����ܼ���ͬ�Ĺ�����е������ȵ���ռ��1�������������������ͬ��ԭ�ӵ�������ͣ�Oԭ����������Ų��� ��
��
�ʴ�Ϊ�� ��
��
��2��H2O�м۲���ӶԸ���=2+$\frac{1}{2}$����6-2��1��=4�Һ���2���µ��Ӷԣ�����Oԭ�Ӳ���sp3�ӻ�����ռ乹��ΪV�Σ�
�ʴ�Ϊ��sp3��V�Σ�
��3��A��ˮ�������ӻ�Ϊsp3��H3O+�������ӻ�Ϊsp3������ԭ�ӵ��ӻ�����û�иı䣬��A����
B��ˮ����ΪV�ͣ�H3O+Ϊ�����ͣ����еļ��Ƿ����˸ı䣬��B��ȷ��
C��ˮ����ΪV�ͣ�H3O+Ϊ�����ͣ���������״�����˸ı䣬��C��ȷ��
D����ṹ��ͬ�������ʲ�ͬ�����Ļ�ѧ���ʷ����˸ı䣬��D��ȷ��
��ѡBCD��
��4������ɫ����ˮCuSO4�ܽ���H2O�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӣ����ɴ�������ӵ����ӷ���ʽ��Cu2++4H2O=[Cu��H2O��4]2+��[Cu��H2O��4]2+����������Cu2+������ΪH2O����λ��Ϊ4�������ɫ��Һ�м�������������Һ��2OH-+Cu��H2O��4]2+=Cu��OH��2��+4H2O������������ͭ��ɫ��״���������백ˮ�����İ���ͭ�������Cu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-��
�ʴ�Ϊ��Cu2+��H2O��4��������ɫ��״�����������ܽ��γ�����ɫ��Һ��Cu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-��
���� ���⿼����ԭ���ڻ�̬ʱ��������Ų�ͼ���ӻ����ۡ�������֪ʶ�㣬������֪ʶ��������Ǩ�ƵĿ��飬ע�����塢�������ӡ���������Լ���λ�����жϣ���Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | FeO | B�� | Fe3O4 | ||
| C�� | Fe2O3 | D�� | Fe3O4 ��Fe2O3����� |
| ѡ�� | ��ѧ��Ӧ�����ӷ���ʽ | ���� |
| A | AlCl3��Һ�м��������ˮ�� Al3++3NH3•H2O�TAl��OH��3��+3NH4+ | ������Ԫ�صIJ���Ӧ����AlO2- |
| B | ��������ͨ���廯������Һ�У� 3Cl2+2Fe2++4Br-�T6Cl-+2Fe3++2Br2 | ��ȷ |
| C | �ð�ˮ���չ����������� 2NH3•H2O+SO2�T2NH4++SO32-ʮH2O | ��ȷ |
| D | �Ȼ������ˮ�� NH4++2H2O�TH3O++NH3•H2O | �����Ȼ���ܽ���ˮ�������仯������д���ӷ���ʽ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �Ȼ�ѧ����ʽδע���¶Ⱥ�ѹǿʱ����H��ʾ��״���µ����� | |
| B�� | �Ȼ�ѧ����ʽ�и�����ǰ�Ļ�ѧ����������ʾ���Ӹ�����ֻ�������ʵ��� | |
| C�� | ͬһ��ѧ��Ӧ����ѧ��������ͬ����H��ͬ����ѧ��������ͬ��״̬��ͬ����HҲ����ͬ | |
| D�� | ��ѧ��Ӧ���������ջ�ų���������μӷ�Ӧ�����ʵ����ʵ��������� |
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� | �� |
��2���ڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳����Cl-��K+��Mg2+��
��3���ٺ͢���Ԫ���γɵĻ��������Һ��Ԫ�آ�ĵ��ʷ�Ӧ�����ӷ���ʽΪCl2+2Br-=Br2+2Cl-��
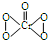 ����˵����ȷ���ǣ�������
����˵����ȷ���ǣ�������| A�� | Cr2O72- ��������CrO5 | |
| B�� | �÷�ӦΪ������ԭ��Ӧ | |
| C�� | ��Ӧ��H2O2����ԭ��H2O | |
| D�� | �˷�Ӧ���ڼ���Cr2O72-���ӵĴ��� |
 O2��g����CO��g������H1 C��s����O2��g����CO2��g������H2
O2��g����CO��g������H1 C��s����O2��g����CO2��g������H2