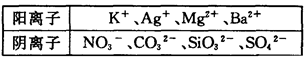��Ŀ����
ij��Һ���ܺ���Cl-��SO42-��CO32-��NH4+��Fe3+��Al3+��K+��ȡ����Һ100mL���������NaOH��Һ�����ȣ��õ�0��02mol���壬ͬʱ�������ɫ���������������ˡ�ϴ�ӡ����գ��õ�1��6g���壻��������Һ�м�������BaCl2��Һ���õ�4��66g����������ij�����
�ش��������⣺
��1��ԭ��Һ��һ�������� ��
��2������ʵ��ͱ�Ҫ�ļ���ȷ����Һ��һ�����ڵ���������Щ ��д����Ҫ��������������̣���
��1��CO32-��2�֣���2��Fe3+��NH4+��SO42-��Cl-��2�֣�
�����������������NaOH��Һ����������ͺ��ɫ������֪��Һ��NH4+��Fe3+��������CO32-����BaCl2��Һ���ɲ���������ij�����֪��Һ�к�SO42-��n(NH3)=0��02mol��֪n(NH4+)=0��02mol��n(Fe2O3)=1��6g/160g��mol-1=0��01mol��֪n(Fe3+)=0��02mol��n(BaSO4)=4��66g/233g��mol-1=0��02mol��֪n(SO42-)=0��02mol����Һ��NH4+��Fe3+���������Ϊ0��02mol+3��0��02mol=0��08mol��SO42-���������Ϊ2��0��02mol=0��04mol���ݵ���غ㣬֪��Һ�б�Ȼ����������һ�������ӣ�������֪ΪCl-������֪��ԭ��Һ��һ����Fe3+��NH4+��SO42-��Cl-��
���㣺������Һ�����ӵĹ�������ӵ��жϣ�ͬʱ�����˵���غ�ԭ�������á�

 ��ǰ����ϵ�д�
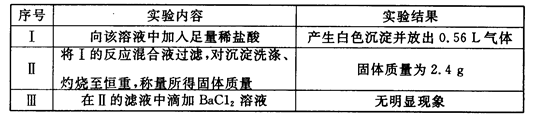
��ǰ����ϵ�д�����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��NH4+��Cl-��Ca2+��Ba2+��CO32-��SO42-. ��ȡ���ݸ�100mL��Һ��������ʵ�飺
��һ�ݼ���AgNO3��Һ�г���������
�ڶ��ݼ�������NaOH��Һ���Ⱥ��ռ���0.08mol���壻
�����ݼ�������BaCl2 ��Һ�õ��������12.54g��������������ϴ�ӡ������������Ϊ4.66g��
��������ʵ�飬�ش��������⣺
��1���ɵ�һ�ݽ��е�ʵ���ƶϸû�����Ƿ�һ������Cl- �� ��ԭ���� .
��2���ɵڶ��ݽ��е�ʵ���֪�������Ӧ���� ���ӣ������ʵ���Ũ��Ϊ .
��3���ɵ����ݽ��е�ʵ���֪12.54g �����ijɷ�Ϊ ��������γɸó�����ԭ������и����ӵ����ʵ�������Ҫ�������̣�
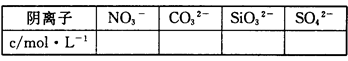
��4���ۺ�����ʵ�飬����Ϊ���½�����ȷ����
| A���û������һ������K+��NH4+��CO32-��SO42-�����ܺ���Cl-����n(K+)��0.04mol |
| B���û������һ������NH4+��CO32-��SO42-�����ܺ���Ca2+�� K+��Cl- |
| C���û������һ������NH4+��CO32-��SO42-�����ܺ���K+��Cl- |
| D���û������һ������NH4+��SO42-�����ܺ���Ca2+��K+��Cl- |
��֪4��ʱ���ֻ�������ˮ�к�Һ���е��ܽ�����±���
| | AgNO3 | Ba(NO3)2 | AgCl | BaCl2 |
| H2O(Һ) | 170g | 9.2g | 1.5��10-4g | 33.3g |
| NH3(Һ) | 86g | 97.2g | 0.8g | 0g |
��������������ˮ���γɸ��ֽⷴӦ�����ӷ���ʽΪ ����Һ�����γɸ��ֽⷴӦ�Ļ�ѧ����ʽΪ ��
 Fe(SCN)2+ K1="200" ��Fe(SCN)2++SCN-
Fe(SCN)2+ K1="200" ��Fe(SCN)2++SCN-