��Ŀ����
��10�֣���1����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L-1��MgCl2��CuCl2�����Һ����μ��백ˮ��������__________�������ѧʽ�������ɸó��������ӷ���ʽΪ____________ ������֪25��ʱKsp[Mg(OH)2]=1.8��10-11,KsP[Cu(OH)2]=2.2��10-20����
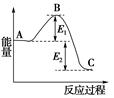
��2������ɴ�ʱ�£�N2H4����NO2��������Դ�����߷�Ӧ����������ˮ��������֪��
��N2��g��+2O2��g��=2NO2��g�� ��H="+67.7" kJ/mol
��N2H4��g��+O2��g��=N2��g��+2H2O��g����H=��534 kJ/mol
���������������Ӧ���Ȼ�ѧ����ʽΪ��
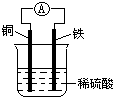
��3���ɴ�ʹ�õ�����ȼ�ϵ�ؾ��и��ܡ����Ͳ���Ⱦ�������ŵ㡣����ȼ�ϵ������ʽ�ͼ�ʽ���֣������ܷ�Ӧ��Ϊ��2H2��O2=2H2O����ʽ����ȼ�ϵ�صĵ������Һ��ǿ����Һ���为���缫��Ӧʽ�ɱ�ʾΪ��2H2��4eһ=4H��������������ӦʽΪ ����ʽ����ȼ�ϵ���еĵ������Һ��ǿ����Һ���������缫��Ӧʽ�ɱ�ʾΪ��O2��2H2O��4eһ=4OHһ�����为����ӦʽΪ
��2������ɴ�ʱ�£�N2H4����NO2��������Դ�����߷�Ӧ����������ˮ��������֪��
��N2��g��+2O2��g��=2NO2��g�� ��H="+67.7" kJ/mol
��N2H4��g��+O2��g��=N2��g��+2H2O��g����H=��534 kJ/mol
���������������Ӧ���Ȼ�ѧ����ʽΪ��
��3���ɴ�ʹ�õ�����ȼ�ϵ�ؾ��и��ܡ����Ͳ���Ⱦ�������ŵ㡣����ȼ�ϵ������ʽ�ͼ�ʽ���֣������ܷ�Ӧ��Ϊ��2H2��O2=2H2O����ʽ����ȼ�ϵ�صĵ������Һ��ǿ����Һ���为���缫��Ӧʽ�ɱ�ʾΪ��2H2��4eһ=4H��������������ӦʽΪ ����ʽ����ȼ�ϵ���еĵ������Һ��ǿ����Һ���������缫��Ӧʽ�ɱ�ʾΪ��O2��2H2O��4eһ=4OHһ�����为����ӦʽΪ
��1������Cu(OH)2�������ѧʽ�������ӷ���ʽΪCu2++2NH3��H2O=Cu(OH)2��+2NH4+��
��2���������������Ӧ���Ȼ�ѧ����ʽΪ��2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g ) ��H=��1135.7kJ/mol
��3��������ӦʽΪO2+4e-+4H+=2H2O��������ӦʽΪH2-2e-+2OH-=2H2O
��2���������������Ӧ���Ȼ�ѧ����ʽΪ��2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g ) ��H=��1135.7kJ/mol
��3��������ӦʽΪO2+4e-+4H+=2H2O��������ӦʽΪH2-2e-+2OH-=2H2O
��1������������Ksp��֪����c(Mg2+)��c(Cu 2+)��ͬ������£� Cu(OH)2�����ӻ����ȳ���Ksp��������������Cu2++2NH3��H2O=Cu(OH)2��+2NH4+
��2���������������Ӧ�Ļ�ѧ����ʽΪ��2N2H4+2NO2=3N2+4H2O���ٸ��ݸ�˹����2���ڣ��ٿ�֪��2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g ) ��H=��1135.7kJ/mol
��3����ʽ����ȼ�ϵ�أ����ܷ�Ӧʽ��ȥ������Ӧʽ�ɵõ�������Ӧʽ��O2+4e-+4H+=2H2O��
��ʽ����ȼ�ϵ�أ����ܷ�Ӧʽ��ȥ�����缫ʽ�ɵø�����Ӧʽ��H2-2e-+2OH-=2H2O
��2���������������Ӧ�Ļ�ѧ����ʽΪ��2N2H4+2NO2=3N2+4H2O���ٸ��ݸ�˹����2���ڣ��ٿ�֪��2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g ) ��H=��1135.7kJ/mol
��3����ʽ����ȼ�ϵ�أ����ܷ�Ӧʽ��ȥ������Ӧʽ�ɵõ�������Ӧʽ��O2+4e-+4H+=2H2O��
��ʽ����ȼ�ϵ�أ����ܷ�Ӧʽ��ȥ�����缫ʽ�ɵø�����Ӧʽ��H2-2e-+2OH-=2H2O

��ϰ��ϵ�д�
�����Ŀ
 ����ȷ����
����ȷ����

 C(g)��D(g)���仯ѧƽ�ⳣ��K���¶ȱ仯�Ĺ�ϵ���±���ʾ���������й��ж���ȷ����
C(g)��D(g)���仯ѧƽ�ⳣ��K���¶ȱ仯�Ĺ�ϵ���±���ʾ���������й��ж���ȷ����