��Ŀ����
16�������£�����A��B��C��D��E��F���ֳ����������֪���ǵ���������K+��Ag+��Ca2+��Ba2+��Fe2+��Al3+����������Cl-��OH-��CH3COO-��NO3-��SO42-��CO32-���ֽ����Ƿֱ����0.1mol/L����Һ����������ʵ�飺����֪�������£���������������ҺŨ��ԼΪ0.00165 g/mL��
�ٲ����ҺA��C��E�ʼ��ԣ��Ҽ���ΪA��E��C��
����B��Һ�еμ�Na2S��Һ������������ǿ�����������Ӹ�����Ϊ1��2�ĺ�ɫ������
����D��Һ�еμ�Ba��NO3��2��Һ������������
����F��Һ�еμӰ�ˮ�����ɰ�ɫ��״����������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��
��������ʵ�����ش��������⣺
��1��д�����л�����Ļ�ѧʽ��ABa��OH��2��BAgNO3��CCa��CH3COO��2��
��2��ʵ����а�ɫ������Ϊ���ɫ��Ӧ�Ļ�ѧ����ʽ��4Fe��OH��2+O2+2H2O=4Fe��OH��3
��3��D��E����Һ��Ӧ���ӷ���ʽ�ǣ�2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����
���� ��֪�������£���������������ҺŨ��ԼΪ0.00165 g/mL����1L��Һ����ຬ��1.65g�����ӣ�������ʵ���Ϊ��$\frac{1.65g}{40g/mol}$=0.04125mol�������ֻ������в����ܺ����������ƣ�
�ٲ����ҺA��C��E�ʼ��ԣ�������ҺΪ��Һ��ˮ��ʼ��Ե���Һ���Ҽ���ΪA��E��C����AΪ���Һ�к��д�����OH-���ӣ�OH-������Ag+��Ca2+��Fe2+��Al3+�����Ӳ��ܴ������棬��Aֻ��ΪBa��OH��2������Խ��Խˮ�⣬EӦΪ̼���Σ��������ӹ��棬ֻ��ΪK2CO3��CΪ�����Σ�
����B��Һ�еμ�Na2S��Һ������������ǿ�����������Ӹ�����Ϊ1��2�ĺ�ɫ�������ú�ɫ����Ϊ��������BΪ��������Һ��
����D��Һ�еμ�Ba��NO3��2��Һ������������˵��B�в���SO42-���ӣ�
����F��Һ�еμӰ�ˮ�����ɰ�ɫ��״����������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��˵��F�к���Fe2+���ӣ�
���Ϸ�����֪��AΪBa��OH��2��BΪAgNO3��CΪCa��CH3COO��2��DΪAlCl3��EΪK2CO3��FΪFeSO4���Դ˽����⣮
��� �⣺��֪�������£���������������ҺŨ��ԼΪ0.00165 g/mL����1L��Һ����ຬ��1.65g�����ӣ�������ʵ���Ϊ��$\frac{1.65g}{40g/mol}$=0.04125mol�������������Ƶ����Ũ��С��0.04125mol/L�������ֻ������в����ܺ����������ƣ�
�ٲ����ҺA��C��E�ʼ��ԣ�������ҺΪ��Һ��ˮ��ʼ��Ե���Һ���Ҽ���ΪA��E��C����AΪ���Һ�к��д�����OH-���ӣ�OH-������Ag+��Ca2+��Fe2+��Al3+�����Ӳ��ܴ������棬��Aֻ��ΪBa��OH��2������Խ��Խˮ�⣬EӦΪ̼���Σ��������ӹ��棬ֻ��ΪK2CO3��CΪ�����Σ�
����B��Һ�еμ�Na2S��Һ������������ǿ�����������Ӹ�����Ϊ1��2�ĺ�ɫ�������ú�ɫ����Ϊ��������BΪ��������Һ��
����D��Һ�еμ�Ba��NO3��2��Һ������������˵��B�в���SO42-���ӣ�
����F��Һ�еμӰ�ˮ�����ɰ�ɫ��״����������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��˵��F�к���Fe2+���ӣ�
���Ϸ�����֪��AΪBa��OH��2��BΪAgNO3��CΪCa��CH3COO��2��DΪAlCl3��EΪK2CO3��FΪFeSO4��
��1�����ݷ�����֪��AΪBa��OH��2��BΪAgNO3��CΪCa��CH3COO��2��
�ʴ�Ϊ��Ba��OH��2��AgNO3��Ca��CH3COO��2��
��2����������������Һ�б�����������������������Ӧ�Ļ�ѧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��3��DΪAlCl3��EΪK2CO3������Ϻ���˫ˮ�ⷴӦ�����������������Ͷ�����̼���壬��Ӧ�����ӷ���ʽΪ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����
�ʴ�Ϊ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����
���� ���⿼�����ʵļ���ͼ��飬Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ���������֮��ķ�Ӧ�����ʵ�����Ϊ���Ĺؼ������ط��������㼰�ƶ������Ŀ��飬ע�����ӹ��漰����غ��Ӧ�ã�

 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�| A�� | ������Fe3+����Һ�У�NH4+��Mg2+��Cl-��HSO3- | |
| B�� | ����Al��H2���ɵ���Һ�У�Na+��NH4+��Cl-��NO3- | |
| C�� | NaOH��Һ�У�K+��Na+��AlO2-��CO32- | |
| D�� | NaHCO3��Һ�У�K+��Al3+��Cl-��SO42- |
| A�� | ���ʱ��ʯī������������������� | |
| B�� | ���ʱ��������Ӧ�ǣ�I--6e-+3H2O�TIO3-+6H+ | |
| C�� | ��Һ������ǿ���ԣ����������� | |
| D�� | ����������Χ��Һ��pH���� |
�ٵ�λʱ��������nmolO2��ͬʱ����2nmolNO2
�ڻ�������ƽ����Է����������ٸı�
��NO2��NO��O2�ķ�Ӧ����֮��Ϊ2��2��1
�ܻ���������ɫ���ٸı�
�ݻ��������ܶȲ��ٸı䣮
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �٢ڢ� | D�� | �ڢܢ� |
| A�� | 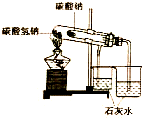 װ�ÿ����ڱȽ�NaHCO3��Na2CO3���ȶ��� | |
| B�� | 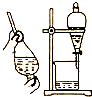 �þƾ���ȡ��ˮ�е����ѡ��װ�� | |
| C�� |  ��ͼװ�ý���ʵ��ɿ�������KMnO4��Һ��ɫ | |
| D�� | 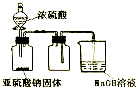 ��ͼװ�ÿ�����ʵ������ȡ���ռ�����SO2 |
2H2��g��+O2��g��=2H2O��l����H=-572kJ•mol-1
H2��g��+0.5O2��g��=H2O��g����H=-242kJ•mol-1
����1molҺ̬ˮ������ʱ���յ������ǣ�������
| A�� | 2.44 kJ | B�� | 44 kJ | C�� | 88 kJ | D�� | 330 kJ |
| A�� | s�����Բ�Σ�p����������� | |
| B�� | CuԪ����Ԫ�����ڱ���ds�� | |
| C�� | 1.5g CH3+�к��еĵ�����Ϊ0.8NA | |
| D�� | DNA�еļ�����������ͨ�������ʵ�ֵ� |
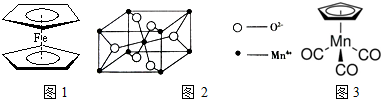
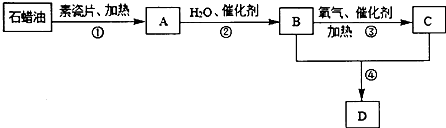
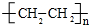 ��
��