��Ŀ����
�ס�����ͬ����ǽ���Ԫ����ɵĵ��ʣ���ͨ��״�����������ɫҺ�塣X��Y��Z�ǻ����X��Һ�ʵ���ɫ��Y��Z����Һ����ʹKSCN��Һ��졣����֮��������ͼ��ʾת����ϵ��

��ش�
��1����Y�ı�����Һ�����ˮ�У��������ȿɵú��ɫҺ�壬��Һ�岻���е�������
A. ����ͨ����Һ��ʱ�γɹ����ġ�ͨ·��
B. ����缫ͨ��ֱ�������һ������Һ����ɫ����
C. ���Һ���м�����������Һ����������
D. ����Һ��������ɡ����պ�������������
��2���ٵ��ʼס��Һ�Y��Һ�е���������������ǿ������˳��Ϊ�� ���û�ѧʽ��ʾ��
���������ʼ�ͨ��X��Һ�з��������ӷ���ʽΪ
��3��ʵ�����Ʊ����ռ���������ļ��ʣ������������£�װ��AΪ����װ�ã���װ��A�������ҩƷ�⣬����װ���й�ѡ���ҩƷ�У�Ũ���ᡢ��ʯ�ҡ����������ס�����ʳ��ˮ������������Һ��
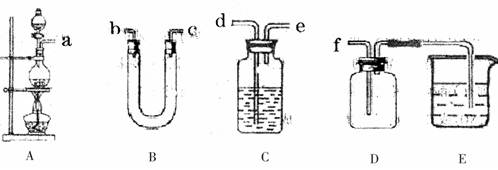
��װ��A�з�����Ӧ�Ļ�ѧ��Ӧ����ʽ��
�ڰ������ķ������Ӹ������ӿڣ�˳��Ϊ
a�� �� �� �� ��f��
��ָ��װ��B��C��E�е�ҩƷ�����ṩ��ҩƷ��ѡ��
B�� C�� E��

��ش�
��1����Y�ı�����Һ�����ˮ�У��������ȿɵú��ɫҺ�壬��Һ�岻���е�������
A. ����ͨ����Һ��ʱ�γɹ����ġ�ͨ·��
B. ����缫ͨ��ֱ�������һ������Һ����ɫ����
C. ���Һ���м�����������Һ����������
D. ����Һ��������ɡ����պ�������������
��2���ٵ��ʼס��Һ�Y��Һ�е���������������ǿ������˳��Ϊ�� ���û�ѧʽ��ʾ��
���������ʼ�ͨ��X��Һ�з��������ӷ���ʽΪ
��3��ʵ�����Ʊ����ռ���������ļ��ʣ������������£�װ��AΪ����װ�ã���װ��A�������ҩƷ�⣬����װ���й�ѡ���ҩƷ�У�Ũ���ᡢ��ʯ�ҡ����������ס�����ʳ��ˮ������������Һ��

��װ��A�з�����Ӧ�Ļ�ѧ��Ӧ����ʽ��
�ڰ������ķ������Ӹ������ӿڣ�˳��Ϊ
a�� �� �� �� ��f��
��ָ��װ��B��C��E�е�ҩƷ�����ṩ��ҩƷ��ѡ��
B�� C�� E��
��1��C��2�֣�
��2����Cl2��Br2��Fe3+(2��) ��Cl2+2Fe2+=2Cl-+2Fe3+(2��)
��3����MnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O(2��)
MnCl2+Cl2��+2H2O(2��)
��a�� d �� e �� b��c �� �� c��b�� ��f��(2��)
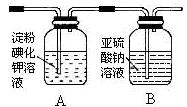
��B�����������ף�1�֣� C������ʳ��ˮ��l�֣�E������������Һ��l�֣�
��2����Cl2��Br2��Fe3+(2��) ��Cl2+2Fe2+=2Cl-+2Fe3+(2��)
��3����MnO2+4HCl(Ũ)
 MnCl2+Cl2��+2H2O(2��)
MnCl2+Cl2��+2H2O(2��)��a�� d �� e �� b��c �� �� c��b�� ��f��(2��)
��B�����������ף�1�֣� C������ʳ��ˮ��l�֣�E������������Һ��l�֣�
��

��ϰ��ϵ�д�
�����Ŀ


 ������
������ �������ϱ��еĿո��С�
�������ϱ��еĿո��С�

 mol��
mol��
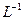 ������100mL����ʵ�������У�
������100mL����ʵ�������У� ��ĸ����
��ĸ���� �У����в�������ȷ���� ������ĸ����
�У����в�������ȷ���� ������ĸ���� ������ƿǰ������Ƿ�©ˮ��
������ƿǰ������Ƿ�©ˮ��