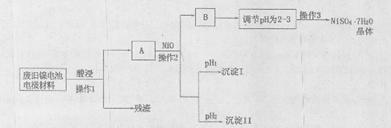
��Ŀ����
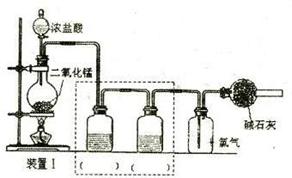
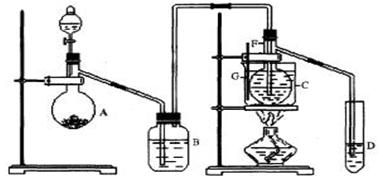
��12�֣����Ȼ���(SCl2)�۵㡪78oC���е�59 oC���ܶ�1.638g/cm3����ˮ�ֽ⣬����������������Ӧ�ϳɶ��Ȼ����ʵ��װ�ã�����F��װ����ˮCaCl2���塣

�Իش��������⣺
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ��
��2��װ��C��ʢ�ŵ��Լ��� ��װ��F������Ϊ
��3��ʵ�鿪ʼǰ���ž�ϵͳ�п�����������Ŀ����
����D�з���һ��������ۣ�����ʹ֮�ڻ���Ȼ��ҡ����ƿʹ��������ƿ�ڱ��γ�һ����Ĥ����������Ŀ����
��4��ʵ��ʱ����η�ֹE��Һ��ӷ�
��5���������߿�����F���ӵ���������ָ������ʢװ�Լ����ƣ������Ƹ�ʵ��װ��

�Իش��������⣺
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ��

��2��װ��C��ʢ�ŵ��Լ��� ��װ��F������Ϊ
��3��ʵ�鿪ʼǰ���ž�ϵͳ�п�����������Ŀ����
����D�з���һ��������ۣ�����ʹ֮�ڻ���Ȼ��ҡ����ƿʹ��������ƿ�ڱ��γ�һ����Ĥ����������Ŀ����
��4��ʵ��ʱ����η�ֹE��Һ��ӷ�
��5���������߿�����F���ӵ���������ָ������ʢװ�Լ����ƣ������Ƹ�ʵ��װ��
��12�֣�
��1��MnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O ��2�֣�
MnCl2+Cl2��+2H2O ��2�֣�
��2��Ũ���� ��ˮ�Ӻ������Eʹ���Ȼ���ֽ⣨��2�֣�
��3����ֹ�������ʱ�������е���������۷�Ӧ�� ����Ӵ�����Լӿ췴Ӧ���ʡ�����1�֣�
��4������ƿ�����ˮ����ȴ��2�֣�
��5��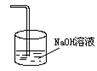 �����а�ȫƿ�Ȳ��۷֣���2�֣�
�����а�ȫƿ�Ȳ��۷֣���2�֣�
��1��MnO2+4HCl(Ũ)
 MnCl2+Cl2��+2H2O ��2�֣�
MnCl2+Cl2��+2H2O ��2�֣���2��Ũ���� ��ˮ�Ӻ������Eʹ���Ȼ���ֽ⣨��2�֣�
��3����ֹ�������ʱ�������е���������۷�Ӧ�� ����Ӵ�����Լӿ췴Ӧ���ʡ�����1�֣�
��4������ƿ�����ˮ����ȴ��2�֣�
��5��
 �����а�ȫƿ�Ȳ��۷֣���2�֣�
�����а�ȫƿ�Ȳ��۷֣���2�֣���

��ϰ��ϵ�д�
�����Ŀ

 Ϊ ��ʵ����Ϊ�˼�����Ӧ���ʣ����� ����ˮ����ʵ���в���������������ŵ���ζ��������_________________�����Լ������Գ�ȥ��
Ϊ ��ʵ����Ϊ�˼�����Ӧ���ʣ����� ����ˮ����ʵ���в���������������ŵ���ζ��������_________________�����Լ������Գ�ȥ�� �����������������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺
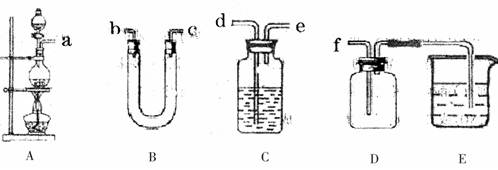
�����������������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺
 ������ĸ��
������ĸ��
 ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���
ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���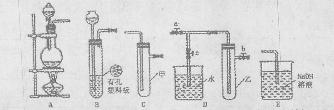
 HgO���ڵĸ��Լ�������____________________����HgO�ӷ�F�еIJ���������________________
HgO���ڵĸ��Լ�������____________________����HgO�ӷ�F�еIJ���������________________