��Ŀ����
����Ҫ0.1mol/LNaOH��Һ450mL��������Һ����������ش��������⣺
�� ʵ���в���Ҫ�������У�
A.������ƽ B. �ձ� C. 500 mL����ƿ D.������ E.�ι� F.����̨ G.��ƿ H.�ƾ���
�� ���ݼ����֪������NaOH������Ϊ g
�� ���в���������Ũ���к�Ӱ�죨��д��ĸ��
ƫ����� ��ƫС���� ����Ӱ����� ��
A������ʱ������������룻
B����NaOH����ֽ���ϳ�����
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У�
D��������ƿת��ʱ��������Һ�彦��
E��δϴ���ܽ�NaOH���ձ�
F������ʱ���ӿ̶���
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ����ټ�ˮ��
H������ƿ�ײ�δ���T����������Һ
�� ʵ���в���Ҫ�������У�
A.������ƽ B. �ձ� C. 500 mL����ƿ D.������ E.�ι� F.����̨ G.��ƿ H.�ƾ���
�� ���ݼ����֪������NaOH������Ϊ g
�� ���в���������Ũ���к�Ӱ�죨��д��ĸ��
ƫ����� ��ƫС���� ����Ӱ����� ��
A������ʱ������������룻
B����NaOH����ֽ���ϳ�����
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У�
D��������ƿת��ʱ��������Һ�彦��
E��δϴ���ܽ�NaOH���ձ�
F������ʱ���ӿ̶���
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ����ټ�ˮ��
H������ƿ�ײ�δ���T����������Һ
��1��F��G��H
��2��1.8
��3��A��C�� B��D��E��F�� G��H��
��2��1.8
��3��A��C�� B��D��E��F�� G��H��
��

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����Ŀ
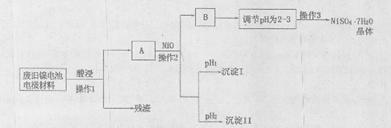
 ����CuCO3��Cu(OH)2��������������SiO2�����Ļ����ʵ�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O�IJ������£�
����CuCO3��Cu(OH)2��������������SiO2�����Ļ����ʵ�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O�IJ������£�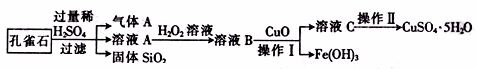
 �����ڴ˲����е���Ҫ������ ��
�����ڴ˲����е���Ҫ������ ��
 �ܶ�Ϊ1.84 g��mL-1��_____________mL��
�ܶ�Ϊ1.84 g��mL-1��_____________mL�� ��һ��ʢ����������ˮ����Ͳ��ϡ�ͣ�����ȴ�����£�
��һ��ʢ����������ˮ����Ͳ��ϡ�ͣ�����ȴ�����£� �����ո����Բ��ӣ���
�����ո����Բ��ӣ���
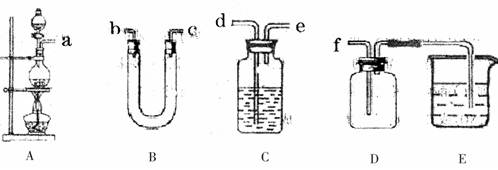
 ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���
ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���