题目内容
6.实验室配制500mL 0.1mol/L Na2CO3溶液,回答下列问题:(1)应用托盘天平称取石碱晶体(十水碳酸钠)14.3g;
(2)配制Na2CO3溶液时需要使用的主要仪器有托盘天平、烧杯、钥匙、500mL容量瓶、玻璃棒、胶头滴管
(3)若实验遇到下列情况,溶液的浓度是偏高,偏低还是不变?
①加水时超过刻度线,再用滴管吸出直至刻度线偏低;
②忘记将洗涤液加入容量瓶偏低;偏低
③容量瓶内壁附有水珠而未干燥处理不变;
④定容后摇匀,液面低于刻度线不变.
分析 (1)根据n=C•V和m=n•M来计算;
(2)根据配制溶液的实验操作过程选择所需的仪器;
(3)分析操作对溶质物质的量、溶液体积的影响,根据c=判断对所配溶液浓度的影响.
解答 解:(1)配制500ml 0.1mol•L-1的Na2CO3溶液需要的Na2CO3的物质的量n=C•V=0.5L×0.1mol•L-1=0.05mol,质量m=n•M=0.05mol×286g/mol=14.3g,
故答案为:14.3;
(2)操作步骤有计算、称量、溶解、移液、洗涤移液、定容、摇匀等操作,一般用托盘天平称量,用药匙取用药品,在烧杯中溶解(可用量筒量取水加入烧杯),并用玻璃棒搅拌,加速溶解.冷却后转移到500mL容量瓶中,并用玻璃棒引流,洗涤烧杯、玻璃棒2-3次,并将洗涤液移入容量瓶中,加水至液面距离刻度线1~2cm时,改用胶头滴管滴加,最后定容颠倒摇匀.所用仪器有托盘天平、烧杯、玻璃棒、500mL容量瓶、胶头滴管、药匙,
故答案为:500mL容量瓶、玻璃棒、胶头滴管;
(3)①加水超过了刻度线,溶液的体积偏大,所配溶液的浓度偏低,溶液是均匀的,取出水使液面恰好到刻度线,剩余溶液的浓度与原溶液的浓度相等,故偏低,
故答案为:偏低;
②忘记将洗涤液加入容量瓶酸,移入容量瓶内溶质的物质的量减小,所配溶液的浓度偏低,
故答案为:偏低;
③定容需要加水,容量瓶内壁附有水珠而未干燥处理,对所配溶液的浓度无影响,
故答案为:不影响;
④溶液配制需加水定容,容量瓶没有干燥,对所配溶液的浓度无影响,
故答案为:不影响.
点评 本题考查一定物质的量浓度溶液的配制,难度不大,根据c=判断理解溶液配制原理与误差分析.

练习册系列答案
相关题目
14.如图所示,把试管放人盛有25℃饱和石灰水的烧杯中,试管中开始放入固体试剂A,再在试管中用滴管滴入5mL液体试剂B.可见到烧杯中饱和的澄清石灰水变浑浊.试回答下列问题:
试推测试剂A和B各是什么?(不一定填满,最少2组)

试推测试剂A和B各是什么?(不一定填满,最少2组)
| 固体试剂A | 液体试剂B | |
| ① | ||
| ② | ||
| ③ | ||
| ④ |

15.下列说法或表示方法中正确的是( )
| A. | 氢气的燃烧热为285.8kJ•mol-1,则氢气燃烧的热化学方程式为:2H2(g)+O2(g)═2H2O(l)△H=-285.8 kJ•mol-1 | |
| B. | 已知中和热为57.3 kJ•mol-1,若将1L1mol•L-1醋酸与含1molNaOH溶液混合,放出的热量要小于57.3kJ | |
| C. | Ba(OH)2•8H2O(s)+2NH4Cl(s)═BaCl2(s)+2NH3(g)+10H2O(l)△H<0 | |
| D. | 等质量的硫蒸气和硫黄分别完全燃烧,后者放出的热量多 |
1.在有机物分子中,若某个碳原子连接着四个不同的原子或原子团,这种碳原子称为“手性碳原子”.凡有一个手性碳原子的物质一定具有光学活性,物质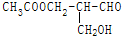 有光学活性,发生下列反应后生成的有机物无光学活性的是( )
有光学活性,发生下列反应后生成的有机物无光学活性的是( )
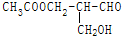 有光学活性,发生下列反应后生成的有机物无光学活性的是( )
有光学活性,发生下列反应后生成的有机物无光学活性的是( )| A. | 与甲酸发生酯化反应 | B. | 与NaOH水溶液共热 | ||
| C. | 与银氨溶液作用 | D. | 在与Br2作用 |
18.CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O 的平衡体系中,加入H218O,一段时间后,则18O存在于( )
| A. | 只存在于乙酸分子中 | B. | 只存在于乙醇分子中 | ||
| C. | 只存在于乙醇、乙酸乙酯分子中 | D. | 只存在于乙酸、乙酸乙酯分子中 |
15.下列化学方程式或离子方程式,不正确的是( )
| A. | 溴乙烷与氢氧化钠的醇溶液共热:CH3CH2Br+NaOHCH2=CH2↑+NaBr+H2O | |
| B. | 用银氨溶液检验乙醛中的官能团:CH3CHO+2[Ag(NH3)2]++2OH-CH3COO-+NH4++3NH3+2Ag↓+H2O | |
| C. | 向苯酚钠溶液中通入少量的CO2:CO2+H2O+2C6H5O-→2C6H5OH+CO32- | |
| D. | 甘氨酸与氢氧化钠溶液反应:H2N-CH2COOH+OH-→H2N-CH2COO-+H2O |
16.下列气体中,溶于水后没有强酸生成的是( )
| A. | Cl2 | B. | SO2 | C. | SO3 | D. | NO2 |
 .
. 和
和
 和
和