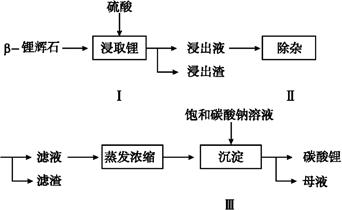
��Ŀ����
��֪��BaSO4(s) + 4C(s) 4CO(g) + BaS(s)��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��Ϊԭ�ϣ�ͨ���������������Ȼ������壨BaCl2��nH2O����
4CO(g) + BaS(s)��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��Ϊԭ�ϣ�ͨ���������������Ȼ������壨BaCl2��nH2O����
�ش��������⣺
��1��������A�Ļ�ѧʽ��_________������ʵ���ҽ��б���ʱ��������������Ĵ���������
a.��NaOH��Һ���� b.��Ũ�������� c.��ȼ
��2���õ�λ�����Һ����������������������ʾ��Ũ�Ƚ�����-���Ũ�ȣ�������g/L��ʾ,����38%��Ũ�������ƺ�����109.5g/L��ϡ����500mL,����Ҫ�IJ����������˲��������� ��
��3��������Ӧ�����ӵ��Լ�R�����������Լ��е�
a.NaOH��Һ b.BaO���� c.��ˮ d.��ʯ��
֤�������Ѿ���ȫ�ķ�����________________________________________________________��
��4�����һ��ʵ��ȷ����Ʒ�Ȼ������壨BaCl2��nH2O���е�nֵ����������ʵ�鲽�裺
�ٳ�����Ʒ��_______ ������_________�����������ƣ�����ȴ �ܳ��� �ݺ��ز�����
���ز�����ָ____________________________________________ _��
�ڢ۲���Ʒ֮���Է��ڸ������н���ʵ���ԭ���� ��
��5�����ؾ�ʯ����̼�Լ��Ȼ��ƹ�ͬ���գ�����ֱ�ӵõ��Ȼ������÷�Ӧ�Ļ�ѧ����Ϊ
BaSO4+ 4C+CaCl2 4CO + CaS+ BaCl2�������������дӱ��պ�Ĺ����з���õ��Ȼ��������ʵ�����̵���ƣ���֪�Ʋ�����ˮ�����������ᣩ��
4CO + CaS+ BaCl2�������������дӱ��պ�Ĺ����з���õ��Ȼ��������ʵ�����̵���ƣ���֪�Ʋ�����ˮ�����������ᣩ��
����������д�������ƣ�
��1��SiO2 c
��2���ձ� 500mL����ƿ ��ͷ�ι�
��3��b��ȡ�ϲ���Һ��С�Թ��У���������������Һ��������������˵��������ȫ��
��4�����ȡ����������ٽ��м��ȡ���ȴ��������ֱ���������γ����Ľ��������0.001gΪֹ����ֹ��ȴ���������տ����е�ˮ���������ʵ����
��5��
���������������1����ҵ�����ؾ�ʯ�����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��������ݵ���SiO2��������֪�������Ļ�ѧ����ʽ��֪������������ΪCO������CO�ķ����ǽ�CO��ȼ��
��2��������Һ���貣�������У��ձ���500mL����ƿ����ͷ�ιܡ���������
��3��BaO��ˮ��Ӧ���������������ܹ��������ӳ������Ҳ������µ����ʣ���ѡ�õ��Լ�ΪBaO���壻֤�������Ѿ���ȫ�ķ�����ȡ�ϲ���Һ��С�Թ��У���������������Һ��������������˵��������ȫ��
��4����ʵ���ԭ�������ü��Ⱥ������ǰ�����������Ȼ��������е�ˮ�����ʵ�����ʵ��������¢ٳ�����Ʒ�ڼ��Ȣ۸���������ȴ �ܳ��� �ݺ��ز��������ز�����ֱָ���������γ����Ľ��������0.001gΪֹ���ڢ۲���Ʒ֮���Է��ڸ������н���ʵ���ԭ����Ϊ�˷�ֹ��ȴ���������տ����е�ˮ���������ʵ����
��5������BaSO4+ 4C+CaCl2 4CO + CaS+ BaCl2��֪��Ӧ��һ����̼�ݳ���Ҫ��ӱ��պ�Ĺ����з���õ��Ȼ���������Ҫ�ӻ����Һ�з���CaS����CaS����ˮ����ֱ�ӹ��˼��ɵõ�BaCl2��Һ��ʵ��������£�
4CO + CaS+ BaCl2��֪��Ӧ��һ����̼�ݳ���Ҫ��ӱ��պ�Ĺ����з���õ��Ȼ���������Ҫ�ӻ����Һ�з���CaS����CaS����ˮ����ֱ�ӹ��˼��ɵõ�BaCl2��Һ��ʵ��������£�
���㣺���ƶϡ�ʵ��������ʹ�á�ʵ���Լ���ѡ��ʵ�����

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�CoCl2��6H2O��һ������Ӫ��ǿ������һ������ˮ�ܿ�(��Ҫ�ɷ�ΪCo2O3��Co(OH)3����������Fe2O3��Al2O3��MnO��)��ȡCoCl2��6H2O�Ĺ����������£�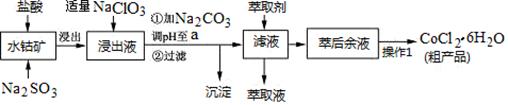
��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±���(��������Ũ��Ϊ��0.01mol/L)
| ������ | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
| ��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��CoCl2��6H2O�۵�Ϊ86�棬������110~120��ʱ��ʧȥ�ᾧˮ������ˮ�Ȼ��ܡ�
��1��д������������Co2O3������Ӧ�����ӷ���ʽ________________________��
��2��д��NaClO3������Ӧ����Ҫ���ӷ���ʽ_____________________________������������Һ���мӹ���NaClO3ʱ�����ܻ������ж����壬д�����ɸ��ж���������ӷ���ʽ_______________��
��3������Na2CO3��pH��a��,�������õ��ij����ɷ�Ϊ ��
��4��������1���а���3������ʵ�����������������_________��__________���ˡ��Ƶõ�CoCl2��6H2O�ں��ʱ���ѹ��ɵ�ԭ����__________________��
��5����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ������Һ���м�����ȡ����Ŀ����_________����ʹ�õ����pH��Χ��________________��

A��2.0~2.5 B��3.0~3.5 C��4.0~4.5 D��5.0~5.5
��6��Ϊ�ⶨ�ֲ�Ʒ��CoCl2��6H2O��������ȡһ�������Ĵֲ�Ʒ����ˮ����������AgNO3��Һ�����ˡ�ϴ�ӣ���������ɺ����������ͨ�����㷢�ֲִ�Ʒ��CoCl2��6H2O��������������100������ԭ�������_____________________������һ�����ɣ�
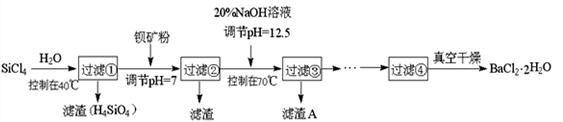
�����(LiCoO2)����ӵ����һ��Ӧ�ù㷺�����͵�Դ��ʵ���ҳ������÷Ͼ����������ӵ�ػ�����������ͭ���ܡ��Ԫ�أ�ʵ��������£�
(1)����ݹ����У������ܽ�����ӷ���ʽΪ__________________________
(2)��ҺA�м���������Һ��ʹCoԪ����CoC2O4��2H2O������ʽ���������������Ʊ������ܼ��ܷ۵���Ҫԭ�ϡ��ڿ�����CoC2O4��2H2O���ȷֽ�ʧ�����ݼ��±����벹���������е��ȷֽⷽ��ʽ��
| ��� | �¶ȷ�Χ/�� | �ȷֽⷽ��ʽ | ����ʧ���� |
| �� | 120��220 | | 19.67% |
| �� | 280��310 | | 56.10% |
(3)����Li2CO3ʱ������Һ�����������ǣ���ʵ������ĽǶȸ������ֿ��ܵ�ԭ��_____________________________________________________________
(4)�������õ�FeCl3��Һ������ˮ�����Խ�����ӷ���ʽ�����侻ˮԭ��________________________________________________________