��Ŀ����
��14�֣� A��B��C��D��E��FΪ������ԭ���������ε���������Ԫ�أ� B��C��D��E��F�ļ����ӵĵ��Ӳ�ṹ��ͬ��A��Eͬ���壬��ԭ�ӵ������������Ĺ�ϵΪA+D=B+F=8����ش�
(1)��д��B2���ʵĵ���ʽ�� ��
��д��������Ԫ����ɵ�4��ԭ�Ӻ˹���18���ӽṹ�����ʵĻ�ѧʽ
(2)��FԪ�ض�Ӧ�Ľ��������ӵ���Һ�еμӹ���E������������Ӧˮ�������Һ��Ӧ�������ӷ���ʽ�� ��
(3)��֪B2A4��BA3�������Ƶ����ʣ�B2A4ͨ�����ȵ�����ͭ��ĩ����ĩ�ɺ�ɫ��Ϊ��ɫ���Ҳ���Դ�������Ⱦ���仯ѧ��Ӧ����ʽ�� ��
(4)��2L���ܱ������У�ͨ��2molB2�����3molA2���壬һ���¶��·�Ӧ����BA3���壬����Ӧ�ﵽƽ��ʱ��A2��Ũ��Ϊ0. 15mol��L-1��ͬʱ�ų�Լ83.2 kJ���������÷�Ӧ���Ȼ�ѧ����ʽ ��
(5)��֪ij������EB3��ˮ���Է�Ӧ�����������嵥�ʺ�һ�ּ��д���仯ѧ����ʽ
����0.1mol�û�������ȫ��Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ ��
(1)��д��B2���ʵĵ���ʽ�� ��
��д��������Ԫ����ɵ�4��ԭ�Ӻ˹���18���ӽṹ�����ʵĻ�ѧʽ
(2)��FԪ�ض�Ӧ�Ľ��������ӵ���Һ�еμӹ���E������������Ӧˮ�������Һ��Ӧ�������ӷ���ʽ�� ��
(3)��֪B2A4��BA3�������Ƶ����ʣ�B2A4ͨ�����ȵ�����ͭ��ĩ����ĩ�ɺ�ɫ��Ϊ��ɫ���Ҳ���Դ�������Ⱦ���仯ѧ��Ӧ����ʽ�� ��
(4)��2L���ܱ������У�ͨ��2molB2�����3molA2���壬һ���¶��·�Ӧ����BA3���壬����Ӧ�ﵽƽ��ʱ��A2��Ũ��Ϊ0. 15mol��L-1��ͬʱ�ų�Լ83.2 kJ���������÷�Ӧ���Ȼ�ѧ����ʽ ��
(5)��֪ij������EB3��ˮ���Է�Ӧ�����������嵥�ʺ�һ�ּ��д���仯ѧ����ʽ
����0.1mol�û�������ȫ��Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ ��
��1�� �� H2O2 ��2��Al3+ + 4OH- ="=" AlO2- + 2H2O
�� H2O2 ��2��Al3+ + 4OH- ="=" AlO2- + 2H2O
(3) N2H4 + 2CuO ="=" 2Cu + N2 + 2H2O ��4��N2(g)+ 3 H2(g) ="=" 2NH3(g) ��H=" -" 92.4KJ/mol
��5��2NaN3+2H2O="=" 3N2��+ H2��+2NaOH 0.1mol��©д��λ�����֣�
 �� H2O2 ��2��Al3+ + 4OH- ="=" AlO2- + 2H2O
�� H2O2 ��2��Al3+ + 4OH- ="=" AlO2- + 2H2O (3) N2H4 + 2CuO ="=" 2Cu + N2 + 2H2O ��4��N2(g)+ 3 H2(g) ="=" 2NH3(g) ��H=" -" 92.4KJ/mol
��5��2NaN3+2H2O="=" 3N2��+ H2��+2NaOH 0.1mol��©д��λ�����֣�
����Ԫ�صĽṹ�����ʿ�֪��A��B��C��D��E��F�ֱ���H��N��O��F��Na��Al��
��1�����������к�������������ʽΪ ��4��ԭ�Ӻ˹���18���ӵķ���Ӧ����˫��ˮ����ѧʽΪH2O2��
��4��ԭ�Ӻ˹���18���ӵķ���Ӧ����˫��ˮ����ѧʽΪH2O2��
��2���������������������������������������Һ�У����Է���ʽΪAl3+ + 4OH- ="=" AlO2- + 2H2O��
��3����ĩ�ɺ�ɫ��Ϊ��ɫ��˵������ͭ����ԭ�������˵���ͭ������Դ�������Ⱦ���������������ǵ�������˷���ʽΪN2H4 + 2CuO ="=" 2Cu + N2 + 2H2O��
A2��Ũ��Ϊ0. 15mol��L-1��������������3mol��0.3mol��2.7mol�����Ե�����3mol����ʱ�ų���������83.2 kJ��2.7��3��92.4kJ������Ȼ�ѧ����ʽΪN2(g)+ 3 H2(g) ="=" 2NH3(g) ��H=" -" 92.4KJ/mol��
��5�����ݻ�����Ļ�ѧʽNaN3��֪���������嵥�ʺ�һ�ּ�ֱ�Ӧ�����������������������ƣ����Է���ʽΪ2NaN3+2H2O="=" 3N2��+ H2��+2NaOH���ڷ�Ӧ��������ˮ�����Ե�����0.1mol�û����ת�Ƶ�����0.1mol��
��1�����������к�������������ʽΪ
 ��4��ԭ�Ӻ˹���18���ӵķ���Ӧ����˫��ˮ����ѧʽΪH2O2��
��4��ԭ�Ӻ˹���18���ӵķ���Ӧ����˫��ˮ����ѧʽΪH2O2����2���������������������������������������Һ�У����Է���ʽΪAl3+ + 4OH- ="=" AlO2- + 2H2O��
��3����ĩ�ɺ�ɫ��Ϊ��ɫ��˵������ͭ����ԭ�������˵���ͭ������Դ�������Ⱦ���������������ǵ�������˷���ʽΪN2H4 + 2CuO ="=" 2Cu + N2 + 2H2O��
A2��Ũ��Ϊ0. 15mol��L-1��������������3mol��0.3mol��2.7mol�����Ե�����3mol����ʱ�ų���������83.2 kJ��2.7��3��92.4kJ������Ȼ�ѧ����ʽΪN2(g)+ 3 H2(g) ="=" 2NH3(g) ��H=" -" 92.4KJ/mol��
��5�����ݻ�����Ļ�ѧʽNaN3��֪���������嵥�ʺ�һ�ּ�ֱ�Ӧ�����������������������ƣ����Է���ʽΪ2NaN3+2H2O="=" 3N2��+ H2��+2NaOH���ڷ�Ӧ��������ˮ�����Ե�����0.1mol�û����ת�Ƶ�����0.1mol��

��ϰ��ϵ�д�
�����Ŀ
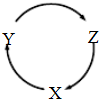
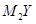 ������˵����ȷ����( )
������˵����ȷ����( )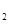 ��M
��M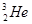 ��
��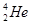 �����ʾ���ͬ
�����ʾ���ͬ