��Ŀ����
15��U��W��X��Y��Z���Ƕ�����Ԫ�أ���ԭ������������������U��W���γ������η���A��U��X���γɳ����³�Һ̬�ķ���B��A��B��Ϊ10���ӷ��ӣ�X����W�γ����ֳ��������ӻ�����ס��ң��仯ѧʽ��ԭ�Ӹ��������ң�YԪ��ԭ�ӵ�K���������M���������ͬ��ZԪ���ǵؿ��к������Ľ�������ش��������⣺��1��ZԪ�������ڱ��е�λ�õ������ڢ�A�壮U��W��X��Y��Z����Ԫ�ص�ԭ�Ӱ뾶��С�����˳����O��N��Al��Mg��Na����Ԫ�ط��ű�ʾ����
��2��U��W�γɵ�18���ӻ�����ĵ���ʽ��
 ��ʵ������ȡ����������A�ķ�����2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ���������ǰ�����
��ʵ������ȡ����������A�ķ�����2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ���������ǰ�������3��A��W������������ˮ���ﷴӦ���ò����ˮ��Һ�����ԣ���ᡱ��������С����������ӷ���ʽ˵����ԭ����NH4++H2O?NH3•H2O+H+������������Ũ�ȴ������������ӵ�Ũ�ȣ�������Һ�����ԣ�
��4����26.4g����Z���ʵĻ����Ͷ�������B�У�������ȫ�ܽ⣬������Һ�н���һ�����ʣ�д���仯ѧʽNaAlO2�������������в����������ڱ�״�������Ϊ15.68L��
��5������Y���������������̼��ȫ��Ӧ���ɵ�ȫ����������������Ũ�����ϣ���ַ�Ӧ�����ж�����̼�Ͷ���������aL����״����������NO2����ת����N2O4������Y���ʵ�������$\frac{3a}{7}$g���ú�a�Ĵ���ʽ��ʾ����
���� U��W��X��Y��Z���Ƕ�����Ԫ�أ���ԭ������������������U��W���γ������η���A����UΪH��WΪN��AΪNH3��U��X���γɳ����³�Һ̬�ķ���B��A��B��Ϊ10���ӷ��ӣ���XΪO��BΪH2O��X����W�γ����ֳ��������ӻ�����ס��ң��仯ѧʽ��ԭ�Ӹ��������ң�����W��Na�����м��ǹ������ƣ����������ƣ�YԪ��ԭ�ӵ�K���������M���������ͬ����YΪMg��ZԪ���ǵؿ��к������Ľ�������ZΪAl��Ȼ������Ԫ�ؼ��䵥�ʡ�����������������
��� �⣺U��W��X��Y��Z���Ƕ�����Ԫ�أ���ԭ������������������U��W���γ������η���A����UΪH��WΪN��AΪNH3��U��X���γɳ����³�Һ̬�ķ���B��A��B��Ϊ10���ӷ��ӣ���XΪO��BΪH2O��X����W�γ����ֳ��������ӻ�����ס��ң��仯ѧʽ��ԭ�Ӹ��������ң�����W��Na�����м��ǹ������ƣ����������ƣ�YԪ��ԭ�ӵ�K���������M���������ͬ����YΪMg��ZԪ���ǵؿ��к������Ľ�������ZΪAl��
��1������������Ϊ13��λ�ڵ������ڢ�A�壬ԭ�ӵĵ��Ӳ���Խ�࣬�뾶Խ��ͬ����Ԫ�ش�������ԭ�Ӱ뾶�ڼ�С����ԭ�Ӱ뾶ΪO��N��Al��Mg��Na��
�ʴ�Ϊ���������ڢ�A�壻O��N��Al��Mg��Na��
��2��U��X�γɵ�18���ӻ�����ΪH2O2�������ʽΪ ��ʵ�������Ȼ�狀ͼ�ʯ�ҷ�Ӧ��ȡ�������������η�Ӧ�����¼�����Σ���ѧ����ʽΪ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O�����鰱�����������ð�����ˮ��Һ�Լ��ԣ����á�ʹʪ��ĺ�ɫʯ����ֽ��������Ҳ�����ð�����Ũ����������̣�������պ��Ũ����IJ���������ƿ�ڣ��������̡���
��ʵ�������Ȼ�狀ͼ�ʯ�ҷ�Ӧ��ȡ�������������η�Ӧ�����¼�����Σ���ѧ����ʽΪ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O�����鰱�����������ð�����ˮ��Һ�Լ��ԣ����á�ʹʪ��ĺ�ɫʯ����ֽ��������Ҳ�����ð�����Ũ����������̣�������պ��Ũ����IJ���������ƿ�ڣ��������̡���
�ʴ�Ϊ�� ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ���������ǰ�����
��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ���������ǰ�����
��3��A��W������������ˮ���������ᣬ��Ӧ�����������ǿ�������Σ�ˮ����Һ�����ԣ�ˮ�ⷴӦ����ʽΪNH4++H2O?NH3•H2O+H+���ʴ�Ϊ���NH4++H2O?NH3•H2O+H+������������Ũ�ȴ������������ӵ�Ũ�ȣ�������Һ�����ԣ�
��4��26.4g�Ĺ�������������ˮ����2Na2O2+2H2O=4NaOH+O2����2Al+2NaOH+2H2O=2NaAlO2+3H2����Ӧ����Ϊ������Һ�н���һ�����ʣ���������Ϊƫ�����ƣ���n��Na2O2����n��Al��=1��2����������Ƶ����ʵ���Ϊxmol�����������ʵ���Ϊ2xmol������78x+54x=26.4g����x=0.2mol�������������������ʵ���Ϊ0.1mol������Ӧ�������������ʵ���Ϊ��$\frac{0.2��2��3}{2}$=0.6mol��������������������ʵ���Ϊ��0.7mol�����Ϊ��0.7mol��22.4L/mol=15.68L���ʴ�Ϊ��NaAlO2��15.68L��
��5��Mg�������̼�ķ�ӦΪ2Mg+CO2 $\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��C��Ũ���ᷴӦ���ɶ�����̼�Ͷ����������������̼�����ʵ���x���ɵ����غ��֪��x��4=��$\frac{aL}{22.4L/mol}$-x����1�����x=$\frac{a}{22.4��5}$mol��
����2Mg+CO2 $\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��֪��Mg�����ʵ���Ϊ$\frac{a}{22.4��5}$mol��2=$\frac{2a}{22.4��5}$mol��
��Mg������Ϊ$\frac{2a}{22.4��5}$mol��24g/mol=$\frac{3a}{7}$���ʴ�Ϊ��$\frac{3a}{7}$��
���� ���⿼��ԭ�ӽṹ��Ԫ�������ɣ�Ԫ�ص��ƶ��ǽ����Ĺؼ���Ӧ��Ϥ���ӵĹ��͡�10��������������ԭ��Ӧ������ѶȲ���

| A�� | 2HgO$\frac{\underline{\;\;��\;\;}}{\;}$ 2Hg+O2 | B�� | Fe3O4+4CO$\frac{\underline{\;����\;}}{\;}$ 3Fe+4CO2 | ||
| C�� | Fe+CuSO4=Cu+FeSO4 | D�� | 2NaCl�����ڣ�$\frac{\underline{\;����\;}}{\;}$ 2Na+Cl2�� |
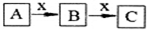 A��B��C��x��Ϊ��ѧ��ѧ�������ʣ���A��B��C����ͬһ��Ԫ�أ���һ�������·�����ͼ��ʾ�Ļ�ѧ�仯����x�������ǣ�������
A��B��C��x��Ϊ��ѧ��ѧ�������ʣ���A��B��C����ͬһ��Ԫ�أ���һ�������·�����ͼ��ʾ�Ļ�ѧ�仯����x�������ǣ�������| A�� | C | B�� | Al | C�� | Fe | D�� | O2 |
| A�� |  ��ϵͳ������2-���� ��ϵͳ������2-���� | B�� | �����Ļ�ѧʽ��KAl��SO4��2•12H2O | ||
| C�� | CH4Si�Ľṹʽ�� | D�� | H2O2�����ģ�ͣ�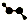 |
| A�� | ��Һ�ȿ��ø�ƿװ������Ӧ�� ��־��װ��Ũ����������۹���Ӧ�� ��־��װ��Ũ����������۹���Ӧ�� ��־ ��־ | |
| B�� | �����Ͻ��ڴ��Ž��յ�������ú��¯ȡů����Ϊ���ɵ�CO��SO2�����������ж� | |
| C�� | ���������һ�������֮ǰ�ŷ��Σ��ȼ��ټӵ��ε���ģ���������������ʹ������ | |
| D�� | �Ͻ���д��ҵʱ��Ǧ�ʷ�����п�ҧ�����������Ǧ�ж� |
| �� | �� | �� | �� | |
| ��Һ | ��ˮ | �������� | ���� | ���� |
| pH | 11 | 11 | 3 | 3 |
| A�� | �ڢ��м���NH4Cl���壬����Һ��$\frac{c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$���� | |
| B�� | �ֱ��ڵ�����Ģڡ�������Һ��ˮϡ��100����������Һ��ˮ�������c��H+����� | |
| C�� | �١�������Һ�������ϣ�������Һ��c��Cl-����c��NH4+����c��OH-����c��H+�� | |
| D�� | �ڡ�������Һ��ϣ���������Һ��pH=7����c��CH3COO-����c��Na+�� |
 ��
��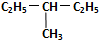 ��
��