��Ŀ����
����Ŀ��������CO2���������ӻ�Ӿ�����ЧӦ��Ϊ�������ŷţ��轫��ҵ�����в�����CO2����������д�������á�
��1��CO2��NH3��Ӧ�ɺϳɻ�������[��ѧʽΪCO(NH2)2]��
��֪��
��2NH3(g)��CO2(g)=NH2CO2NH4(s) ��H=��159.5kJ/mol
��NH2CO2NH4(s)=CO(NH2)2(s)��H2O(g) ��H=��116.5kJ/mol
��H2O(l)=H2O(g) ��H=��44.0kJ/mol
д��CO2��NH3�ϳ����غ�Һ̬ˮ���Ȼ�ѧ��Ӧ����ʽ��______________��
��2��CO2��H2Ҳ�����ںϳɼ״���CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)��������ɱ�ĺ�ѹ�ܱ�������ѹǿΪPʱ���÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ����ƽ��ʱCO2��ת������ͼһ��ʾ��
CH3OH(g)+H2O(g)��������ɱ�ĺ�ѹ�ܱ�������ѹǿΪPʱ���÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ����ƽ��ʱCO2��ת������ͼһ��ʾ��
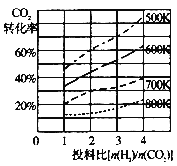
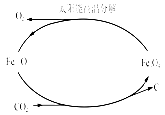
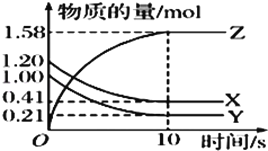
ͼһ ͼ�� ͼ��
���÷�Ӧ��S 0��H 0���������������
��700Kʱ����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��KP= ��
�����¶Ȳ��䣬��С��Ӧ��Ͷ�ϱ�[n(H2)/n(CO2)]��Kֵ �����������С�����䡱����
��700KͶ�ϱ�[n(H2)/n(CO2)] = 2ʱ����ƽ��ʱH2��ת����Ϊ ��
��3������̫���ܺ�ȱ��������[��Fe0.9O]�ɽ�������������CO2�Ƚ�Ϊ̼��������ʵ��CO2����Դ����ת��������ͼ����ʾ������1molȱ��������[Fe0.9O]������CO2��ȫ��Ӧ������ molC(̼)��
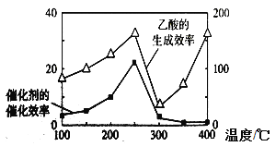
��4����TiO2/Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᡣ�ڲ�ͬ�¶��´����Ĵ�Ч����������������ʵĹ�ϵ��ͼ������ν���ͼ��250-400��ʱ�¶�������������������ʱ仯�Ĺ�ϵ�� ��
���𰸡�
��1��2NH3(g)+CO2(g)=CO(NH2)2(s)+H2O(l) ��H���C87.0kJ/mol��2�֣�
��2��������1�֣� ����1�֣�
��![]() ��2�֣�
��2�֣�
��������2�֣�
��45%��2�֣�
��3��0.1��2�֣�
��4����250-300�������У�������Ӱ�����ʵ���Ҫ���أ������Ĵ�Ч�ʽ��ͣ����·�Ӧ����Ҳ���ͣ�����300-400��ʱ����Ч�ʵ��ұ仯�̶Ƚ�С������Ӧ�������ӽ����ԣ���˸ù������¶���Ӱ�����ʵ���Ҫ���أ��¶�Խ�ߣ���Ӧ����Խ����2�֣�
��������
���������
��1����֪����2NH3(g)��CO2(g)=NH2CO2NH4(s) ��H=��159.5 kJ/mol
��NH2CO2NH4(s)=CO(NH2)2(s)��H2O(g) ��H=��116.5 kJ/mol
��H2O(l)=H2O(g) ��H=��44.0 kJ/mol
����ݸ�˹���ɿ�֪��+���������õ�CO2��NH3�ϳ����غ�Һ̬ˮ���Ȼ�ѧ��Ӧ����ʽΪ2NH3(g)+CO2(g)=CO(NH2)2(s)+H2O(l) ��H���C87.0 kJ/mol��
��2������ӦCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ������ϵ���ڼ�С������S��0����ͼһ��֪���¶�Խ�ߣ�CO2��ת����ԽС��˵���¶����ߣ�ƽ�������ƶ������Ը÷�Ӧ��H��0��
CH3OH(g)+H2O(g) ������ϵ���ڼ�С������S��0����ͼһ��֪���¶�Խ�ߣ�CO2��ת����ԽС��˵���¶����ߣ�ƽ�������ƶ������Ը÷�Ӧ��H��0��
��700Kʱ����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��KP=![]() ��
��
��Kֻ���¶��йأ��¶Ȳ��䣬Kֵ���䡣
��700KͶ�ϱ�[n(H2)/n(CO2)] = 2ʱ��������H2Ϊ2mol��CO2Ϊ1mol ����ʱCO2��ת����Ϊ30%������H2Ϊ0.9mol����ƽ��ʱH2��ת����Ϊ45%��
��3������ͼʾ�õ���ѧ����ʽΪ��Fe0.9O+0.1CO2=xC+0.3Fe3O4������̼ԭ���غ�õ�x=0.1��
��4������ͼ����250-300�������У�������Ӱ�����ʵ���Ҫ���أ������Ĵ�Ч�ʽ��ͣ����·�Ӧ����Ҳ���ͣ�����300-400��ʱ����Ч�ʵ��ұ仯�̶Ƚ�С������Ӧ�������ӽ����ԣ������Ǹù������¶���Ӱ�����ʵ���Ҫ���أ��¶�Խ�ߣ���Ӧ����Խ�ʴ�Ϊ����250-300�������У�������Ӱ�����ʵ���Ҫ���أ���Ϊ�����Ĵ�Ч�ʽ��ͣ����·�Ӧ����Ҳ���ͣ�����300-400��ʱ����Ч�ʵ��ұ仯�̶Ƚ�С������Ӧ�������ӽ����ԣ��ù������¶���Ӱ�����ʵ���Ҫ���أ��¶�Խ�ߣ���Ӧ����Խ��

 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�����Ŀ����ס��ҡ��������ܱ������г���һ������A��B��������Ӧ��xA(g)+B(g)![]() 2C(g)���������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������C��Ũ����ʱ��仯��ϵ�ֱ�����ͼ���±���ʾ��
2C(g)���������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������C��Ũ����ʱ��仯��ϵ�ֱ�����ͼ���±���ʾ��

���� | �� | �� | �� |
�ݻ� | 1L | 1L | 2L |
�¶�/�� | T1 | T2 | T2 |
��Ӧ�� ��ʼ�� | 1molA 2molB | 1molA 2molB | 4molA 8molB |
����˵����ȷ����
A. ��ͼ��֪T1<T2���Ҹ÷�ӦΪ���ȷ�Ӧ
B. ǰ10min�ڼס��ҡ������������з�Ӧ��ƽ������:v(A)��<v(A)��< v(A)��
C. ƽ��ʱA��ת����a��a��<a��<a��
D. T1ʱ�÷�Ӧ��ƽ�ⳣ��K=7.2