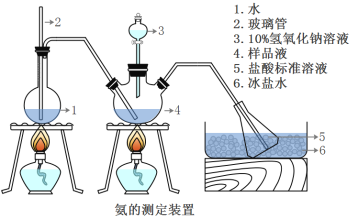
ЬтФПФкШн
ЁОЬтФПЁПАДвЊЧѓаДШШЛЏбЇЗНГЬЪНЃК
ЃЈ1ЃЉ25ЁцЁЂ101kPaЬѕМўЯТГфЗжШМЩевЛЖЈСПЕФC2H2ЦјЬхЗХГіШШСПЮЊ1300kJЃЌОВтЖЈЃЌНЋЩњГЩЕФCO2ЭЈШызуСПГЮЧхЪЏЛвЫЎжаВњЩњ100gАзЩЋГСЕэЃЌаДГіБэЪОC2H2ЦјЬхГфЗжШМЩеЕФШШЛЏбЇЗНГЬЪНЃК___ЃЛ
ЃЈ2ЃЉвбжЊЯЁШмвКжаЃЌ1molH2SO4гыNaOHШмвКЧЁКУЭъШЋЗДгІЪБЃЌЗХГі114.6kJШШСПЃЌаДГіБэЪОH2SO4гыNaOHЗДгІЕФжаКЭШШЕФШШЛЏбЇЗНГЬЪНЃК___ЁЃ
ЁОД№АИЁПC2H2(g)+![]() O2(g)=2CO2(g)+H2O(l) ЁїH=-2600kJ/mol NaOH(aq)+
O2(g)=2CO2(g)+H2O(l) ЁїH=-2600kJ/mol NaOH(aq)+![]() H2SO4(aq)=
H2SO4(aq)=![]() Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/mol
Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/mol
ЁОНтЮіЁП
(1)ИљОнЩњГЩЬМЫсИЦЕФжЪСПМЦC2H2ЕФЮяжЪЕФСПЃЌНсКЯШМЩеШШЕФИХФюЪщаДБэЪОC2H2ШМЩеШШЛЏбЇЗНГЬЪНЃЛ
(2)ИљОнжаКЭШШЕФИХФюЃКЯЁЕФЧПЫсКЭЧПМюЗДгІЩњГЩ1molЫЎЫљЗХГіЕФШШСПЧѓГіжаКЭШШВЂЪщаДГіжаКЭШШЕФШШЛЏбЇЗНГЬЪНЁЃ
(1)n(CaCO3)=![]() =1molЃЌдђИљОнCдЊЫиЪиКуЃЌПЩжЊn(CO2)=1molЃЌМДC2H2ШМЩеЩњГЩЕФЖўбѕЛЏЬМЮЊ1molЃЌгЩC2H2ШМЩеЕФЛЏбЇЗНГЬЪН2C2H2+5O2=4CO2+2H2OПЩжЊЗДгІЕФC2H2ЕФЮяжЪЕФСПЪЧn(C2H2)=
=1molЃЌдђИљОнCдЊЫиЪиКуЃЌПЩжЊn(CO2)=1molЃЌМДC2H2ШМЩеЩњГЩЕФЖўбѕЛЏЬМЮЊ1molЃЌгЩC2H2ШМЩеЕФЛЏбЇЗНГЬЪН2C2H2+5O2=4CO2+2H2OПЩжЊЗДгІЕФC2H2ЕФЮяжЪЕФСПЪЧn(C2H2)=![]() ЃЌвђЮЊШМЩе
ЃЌвђЮЊШМЩе![]() molЕФC2H2ЗХГіЕФШШСПЮЊ1300kJЃЌЫљвдШМЩе1molC2H2ВњЩњCO2ЦјЬхКЭВњЩњвКЬЌЫЎЗХГіШШСПЪЧ2600kJЃЌЙЪC2H2ЕФШМЩеШШЮЊ2600kJЃЌЫљвдБэЪОC2H2ШМЩеШШЛЏбЇЗНГЬЪНЮЊC2H2(g)+
molЕФC2H2ЗХГіЕФШШСПЮЊ1300kJЃЌЫљвдШМЩе1molC2H2ВњЩњCO2ЦјЬхКЭВњЩњвКЬЌЫЎЗХГіШШСПЪЧ2600kJЃЌЙЪC2H2ЕФШМЩеШШЮЊ2600kJЃЌЫљвдБэЪОC2H2ШМЩеШШЛЏбЇЗНГЬЪНЮЊC2H2(g)+![]() O2(g)=2CO2(g)+H2O(l) ЁїH=-2600kJ/molЃЌЙЪД№АИЮЊЃКC2H2(g)+
O2(g)=2CO2(g)+H2O(l) ЁїH=-2600kJ/molЃЌЙЪД№АИЮЊЃКC2H2(g)+![]() O2(g)=2CO2(g)+H2O(l) ЁїH=-2600kJ/molЃЛ
O2(g)=2CO2(g)+H2O(l) ЁїH=-2600kJ/molЃЛ
(2)1mol H2SO4ШмвКгызуСПNaOHШмвКЭъШЋЗДгІЃЌЗХГі114.6kJЕФШШСПЃЌМДЩњГЩ2molЫЎЗХГі114.6kJЕФШШСПЃЌЗДгІЕФЗДгІШШЮЊ-114.6kJ/molЃЌжаКЭШШЮЊ-57.3kJ/molЃЌдђжаКЭШШЕФШШЛЏбЇЗНГЬЪНNaOH(aq)+![]() H2SO4(aq)=
H2SO4(aq)=![]() Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/molЃЌЙЪД№АИЮЊЃКNaOH(aq)+
Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/molЃЌЙЪД№АИЮЊЃКNaOH(aq)+![]() H2SO4(aq)=
H2SO4(aq)=![]() Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/molЁЃ
Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/molЁЃ

ЁОЬтФПЁПЯжгаВПЗжЖЬжмЦкдЊЫиЕФаджЪЛђдзгНсЙЙШчЯТБэЃК
дЊЫиБрКХ | дЊЫиаджЪЛђдзгНсЙЙ |
T | MВуЩЯЕчзгЪ§ЪЧKВуЩЯЕчзгЪ§ЕФ3БЖ |
X | зюЭтВуЕчзгЪ§ЪЧДЮЭтВуЕчзгЪ§ЕФ2БЖ |
Y | ГЃЮТЯТЕЅжЪЮЊЫЋдзгЗжзгЃЌЦфЧтЛЏЮяЫЎШмвКГЪМюад |
Z | дЊЫизюИпе§МлЪЧЃЋ7Мл |
ЃЈ1ЃЉдЊЫиXЮЛгкдЊЫижмЦкБэЕФЕк____жмЦкЕк__зхЃЌЫќЕФвЛжжКЫЫиПЩВтЖЈЮФЮяФъДњЃЌетжжКЫЫиЕФЗћКХЪЧ____ЁЃ
ЃЈ2ЃЉдЊЫиYЕФдзгНсЙЙЪОвтЭМЮЊ___ЃЌгыЧтдЊЫиаЮГЩвЛжжРызгYH4+ЃЌаДГіФГШмвКжаКЌгаИУЮЂСЃЕФМьбщЗНЗЈ_____ЁЃ
ЃЈ3ЃЉдЊЫиZгыдЊЫиTЯрБШЃЌЗЧН№ЪєадНЯЧПЕФЪЧ___(гУдЊЫиЗћКХБэЪО)ЃЌЯТСаБэЪіжаФмжЄУїетвЛЪТЪЕЕФЪЧ___ЁЃ
aЃЎГЃЮТЯТZЕФЕЅжЪКЭTЕФЕЅжЪзДЬЌВЛЭЌ
bЃЎZЕФЧтЛЏЮяБШTЕФЧтЛЏЮяЮШЖЈ
cЃЎвЛЖЈЬѕМўЯТZКЭTЕФЕЅжЪЖМФмгыЧтбѕЛЏФЦШмвКЗДгІ
ЃЈ4ЃЉЬНбАЮяжЪЕФаджЪВювьадЪЧбЇЯАЕФживЊЗНЗЈжЎвЛЁЃTЁЂXЁЂYЁЂZЫФжждЊЫиЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяжаЛЏбЇаджЪУїЯдВЛЭЌгкЦфЫћШ§жжЕФЪЧ____ЃЌРэгЩ______ЁЃ
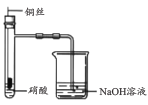
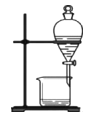
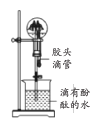
ЁОЬтФПЁПЯТСаЪЕбщзАжУЛђВйзїгыЪЕбщФПЕФВЛЯрЗћЕФЪЧ
|
|
|
|
A. жЄУїЭгыЯѕЫсЕФЗДгІ | B. ЗжРыввДМКЭввЫсЕФЛьКЯвК | C. жЄУїАБЦјвзШмгкЫЎЧвЫЎШмвКГЪМюад | D. ХфжЦ100 mL0.100 mol/LNaClШмвК |
A.AB.BC.CD.D