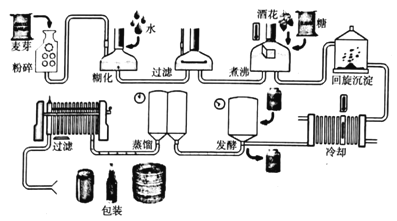
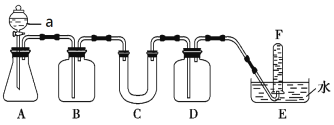
��Ŀ����
����Ŀ���״�����Ҫ�Ļ���ԭ�ϣ��ֿ���Ϊȼ�ϡ����úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ����������ºϳɼ״�������������Ӧ���£�
��CO��g����2H2��g��![]() CH3OH��g����H1
CH3OH��g����H1
��CO2��g����3H2��g��![]() CH3OH��g����H2O��g����H2
CH3OH��g����H2O��g����H2
��CO2��g����H2��g��![]() CO��g����H2O��g����H3
CO��g����H2O��g����H3
�ش��������⣺��֪��Ӧ���е���صĻ�ѧ���������������
��ѧ�� | H��H | C��O |
| H��O | C��H |
E/��kJ��mol��1�� | 436 | 343 | 1076 | 465 | 413 |
��1���ɴ˼�����H1��__kJ��mol��1����֪��H2����58kJ��mol��1������H3��__kJ��mol��1��
��2����һ�������£���2L�����ܱ������г���1molCO2��3molH2������Ӧ����5minʱ���������0.4molH2O����5min�ķ�Ӧ����v��H2��=__��д��һ���ܼӿ췴Ӧ���ʵĴ�ʩ___
��3���״��ǵ綯����������ȼ�ϣ�����ԭ����ͼ��ʾ��
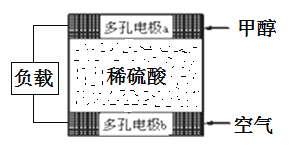
��缫bΪȼ�ϵ�ص�__��������������������������缫a�����ĵ缫��ӦʽΪ��____
���𰸡�-99 ��41 0.12moL/��L��min�� �����¶Ȼ������� �� CH3OH-6e-+H2O=CO2 +6H+
��������
��1����Ӧ��=��Ӧ����ܼ���-��������ܼ��ܣ������H1��![]() �����ݸ�˹���ɣ���Ӧ��-��Ӧ��=��Ӧ�ۣ�����H3��
�����ݸ�˹���ɣ���Ӧ��-��Ӧ��=��Ӧ�ۣ�����H3��![]() ��
��
��2��5minʱ���������0.4molH2O������ˮ��ʾ�Ļ�ѧ��Ӧ����Ϊ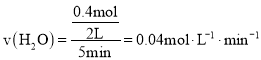 ��5min�ķ�Ӧ����v��H2��= 0.12moL/��L��min�����ܼӿ췴Ӧ���ʵĴ�ʩΪ�����¶Ȼ���������
��5min�ķ�Ӧ����v��H2��= 0.12moL/��L��min�����ܼӿ췴Ӧ���ʵĴ�ʩΪ�����¶Ȼ���������
��3��b�缫Ϊȼ�ϵ�ؽ�������һ��Ϊ������a�缫Ϊ�������缫��ӦʽΪCH3OH-6e-+H2O=CO2 +6H+��

 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�