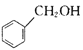
��Ŀ����
����Ŀ������һ���Σ���A��B��C��D��E���ֶ�����Ԫ��Ԫ����ɡ�������ˮ��ɵ�����������ӣ����к�����A��B�γɵ�10���������ӡ�AԪ��ԭ�Ӻ�����������E����1��D��E����ͬ���塣�ü�������ʵ�飺
��ȡ�����ľ�����������ˮ�����Һ��
��ȡ��������Һ���Թ��У������м���ϡ���ᣬ�ټ���BaCl2��Һ�����ְ�ɫ������
��ȡ��������Һ���Թ�����ε���NaOH��Һ�����ɳ��������������NaOH��Һ�������ϵ����ͼ��ʾ��

��ȡ��������Һ���Թ��У���������NaOH��Һ�����ȡ�
�ش��������⣺
��1��C��Ԫ�ط�����_______��D�����ڱ��е�λ����________��
��2�����ⶨ�����Ħ������Ϊ453 gmol-1�����������Ӻ����������ʵ���֮��Ϊ1:1�������Ļ�ѧʽΪ________��
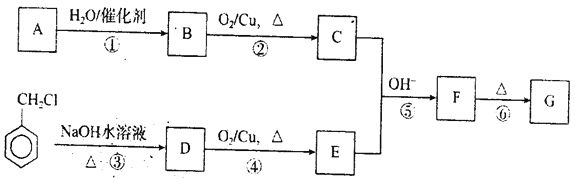
��3��ʵ����и���ͼ���V(oa):V(ab):V(bc)= _______��
��4��ʵ��������ӷ���ʽ��___________��
���𰸡� Al �������ڵ�VIA�� NH4Al(SO4)2��12H2O 3��1��1 NH4����Al3����5OH��![]() NH3����AlO2����3H2O
NH3����AlO2����3H2O
������������A��B��C��D��E���ֶ�����Ԫ����ɵ�һ���Σ����м���Һ�м���ϡ���ᣬ�ټ���BaCl2��Һ�����ְ�ɫ������˵�����к���SO42-�����м������NaOH��Һ�����ȣ��д̼�����ζ���������ɣ�������ΪNH3�������Һ�к���NH4+������ȡ��������Һ����ε���NaOH��Һ�����ɳ�����ʼ�����������������������䣬���γ�����С����ȫ��ʧ�������Һ�϶�����Al3+��������ˮ�������������ΪSO42-��Al3+��NH4+��A��B�γɵ�10����������ΪNH4+��D��Eͬ���壬����Ӧ�γ�SO42-����AԪ��ԭ�Ӻ�����������E����l����AΪNԪ�ء�EΪOԪ�ء�DΪSԪ�ء�BΪHԪ�ء�CΪAl����
��1��C������C��Ԫ�ط�����Al��D��SԪ�أ�λ�����ڱ��е������ڢ�A������2�����ⶨ�����Ħ������Ϊ453g/mol����1mol�����к���12mol�ᾧˮ�����������Ӻ������ӵ�����Է�������Ϊ��453-216=237�������Ӻ����������ʵ���֮��1��1�����ݵ�����ԭ�����仯ѧʽΪ��NH4Al(SO4)2��12H2O����3��ȡ��������Һ���Թ�����ε���NaOH��Һ���ȷ�����Ӧ��Al3++3OH-=Al��OH��3������������Ӧ��NH4++OH-=NH3H2O���������������ܽ⣬������Ӧ��OH-+Al��OH��3=AlO2-+2H2O������NH4Al(SO4)2��12H2OΪ1mol�����������NaOH�ֱ�Ϊ3mol��1mol��1mol������V��Oa����V��ab����V��bc��=3mol��1mol��1mol=3��1��1����4������Һ�м������NaOH��Һ�����ȣ�笠����ӡ������Ӿ����Ժ�������������֮�䷴Ӧ��NH4+��Al3+�����ʵ���֮��Ϊ1��1����Ӧ���ӷ���ʽΪ��NH4����Al3����5OH��![]() NH3����AlO2����3H2O��
NH3����AlO2����3H2O��

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�����Ŀ������[CO(NH2)2]�Ǻ�������ߵĵ��ʡ�
(1) ��֪��ҵ�Ϻϳ����صķ�Ӧ��Ϊ����������
��1����2NH3(I)+CO2(g) ![]() H2NCOONH4(I) H1=-330.0kJ��mol-1
H2NCOONH4(I) H1=-330.0kJ��mol-1
��2����H2NCOONH4(I) ![]() H2O(I)+CO(NH2)2(I) H2=+226.3 kJ��mol-1
H2O(I)+CO(NH2)2(I) H2=+226.3 kJ��mol-1
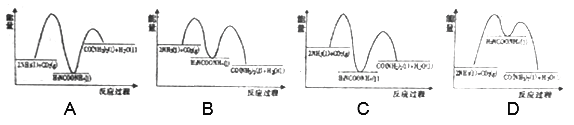
�����и�ͼ����ȷ��ʾ���غϳɹ����������仯���ߵ���___________������ĸ��ţ���
(2)һ�������¹�ҵ�ϳ����ص��ܷ�Ӧ��CO2(g)+2HH3(g) ![]() CO(NH2)2(g)+H2O(g)��t��ʱ�����ݻ��㶨Ϊ2L���ܱ������м���0.20molCO2��0.80molNH3����Ӧ��CO2�����ʵ�����ʱ��仯���±���ʾ��
CO(NH2)2(g)+H2O(g)��t��ʱ�����ݻ��㶨Ϊ2L���ܱ������м���0.20molCO2��0.80molNH3����Ӧ��CO2�����ʵ�����ʱ��仯���±���ʾ��
ʱ��/min | 0 | 40 | 70 | 80 | 100 |
n(CO2)/mol | 0.20 | 0.12 | 0.10 | 0.10 | 0.10 |
��ǰ40min��v(NH3)=_________�����¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ___________��
��30minʱv��(CO2)________80minʱv��(H2O)��ѡ�>������=������<������
����100minʱ�����������������䣬���������г���0.10molCO2��0.4molNH3�����½���ƽ���CO2��ת������ԭƽ����Ƚ�________������������䡱������С����
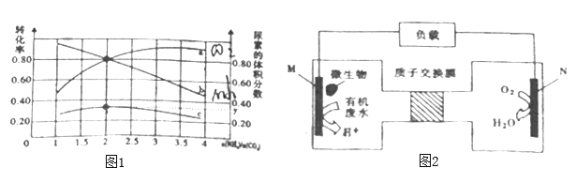
�ܰ�̼��[n(NH3)/n(CO2)]�Ժϳ�������Ӱ�죬���º��������½������ʵ���Ϊ3mol��NH3��CO2�Ļ�����尴��ͬ��̼�Ƚ��з�Ӧ�������ͼ1��ʾ��a��b�߷ֱ��ʾCO2��NH3��ת���ʱ仯��c�߱�ʾƽ����ϵ�����ص���������仯��[n(NH3)/n(CO2)]=______ʱ�����ز����������ͼ��y=_______����ȷ��0.01����
(3)��ҵ������Ϊ������صIJ��ʿɲ�ȡ�Ĵ�ʩ��___________________��
(4)���ڿ�ѧ�ҷ�������ɽ�������ˮ�е�����ֱ��ת��Ϊ�Ի����Ѻõ��������ʣ��乤��ԭ����ͼ2��ʾ���ش��������⣺
��N��Ϊ_________���������������������M�缫��Ӧʽ________________��
��N�����ı�״����33.6L����ʱ��M�������ϴ��������ص�����Ϊ__________g��
����Ŀ��ʵ������ȡ���ᶡ����ʵ��װ�������¼ס�������װ�ÿɹ�ѡ�á�
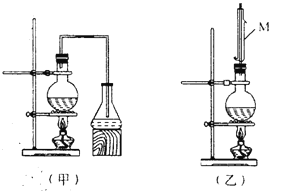
�Ʊ����ᶡ�����漰���й����ʵ��������ʼ��±���
���� | 1-���� | ���ᶡ�� | |
�۵㣨�棩 | 16.6 | -89.5 | -73.5 |
�е㣨�棩 | 117.9 | 117 | 126.3 |
�ܶȣ�g/cm3�� | 1.05 | 0.81 | 0.88 |
ˮ���� | ���� | ���ܣ�9g/100gˮ�� | �� |
��1������M������Ϊ_________��
��2����ȡ���ᶡ��ѡ����װ�ö���ѡ�ü�װ�õ�������__________��
��3�����Ʊ����ᶡ�����õĻ�����з��롢�ᴿ���ᶡ��ʱ����Ҫ�����ಽ����������ͼʾ�IJ����У��϶���Ҫ�Ļ�ѧ������______��ѡ��𰸱�ţ���
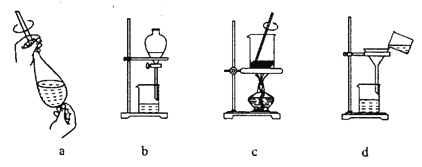
��4���л���ķ�������У�������Ҫʹ�÷�Һ©����������ʹ�÷�Һ©��ǰ����_____����д��������ijͬѧ�ڽ��з�Һ����ʱ��������Һ�����������������ԭ�����Һ©�����������⣬������_________��д��һ�㣩��