��Ŀ����
����Ŀ�������������ž�����ҵ�Ŀ��ٷ�չ�����������������Ȼ���IJ�����Ҳ��֮Ѹ����������ˣ����Ȼ���ת��Ϊ�����ļ�����Ϊ��ѧ�о����ȵ㡣��ش�
(1)��ͼ��ʾ��CuO������HCl�������ķ�Ӧ���̣����ܷ�Ӧ�Ļ�ѧ����Ϊ______��
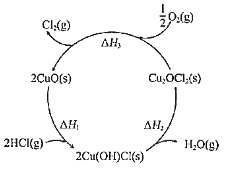
(2)�о�HCl��������Ӧ���¶ȡ�![]() ��
��![]() �����ض�HClת���ʵ�Ӱ�죬�õ�����ʵ������
�����ض�HClת���ʵ�Ӱ�죬�õ�����ʵ������
������Na2S2O3��Һ��KI��Һ�ⶨ��Ӧ����Cl2�����ʵ�����������V1mLc1mol��L-1��Na2S2O3��Һ��������Cl2____________mol(��֪2S2O![]() +I2=S4O
+I2=S4O![]() +2I-)��
+2I-)��
��![]() ��ʾ������������HCl(g)����֮�ȣ��Ǻ�����Ӧ����������Ӵ����������������
��ʾ������������HCl(g)����֮�ȣ��Ǻ�����Ӧ����������Ӵ����������������![]() =4��
=4��![]() =50g��min��mol-1ʱ��ÿ��������1g�������������Ϊ_____L(����Ϊ��״����)��
=50g��min��mol-1ʱ��ÿ��������1g�������������Ϊ_____L(����Ϊ��״����)��
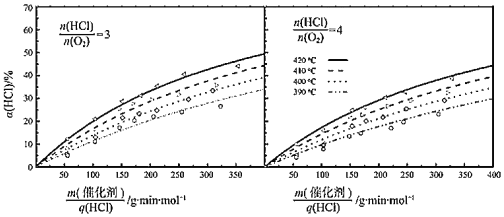
����420����![]() =3��
=3��![]() =200g��min��mol-1�����£���(HCl)Ϊ33.3%����O2�ķ�Ӧ����Ϊ_______mol��g-1��min-1��
=200g��min��mol-1�����£���(HCl)Ϊ33.3%����O2�ķ�Ӧ����Ϊ_______mol��g-1��min-1��
�ܱȽ����������ַ�Ӧ������O2�ķ�Ӧ���ʣ�v��_______v��(����������=����������)��
��.410����![]() =3��
=3��![]() =350g��min��mol-1��
=350g��min��mol-1��
��.390����![]() =4��
=4��![]() =350g��min��mol-1��
=350g��min��mol-1��
(3)��101.325kPaʱ���Ժ�N2��HCl��O2�Ļ������ⶨ��ͬ�¶���HCl��������Ӧ��HCl��ƽ��ת���ʣ��õ���ͼ���
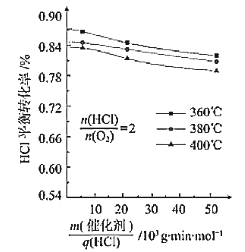
��360��ʱ��Ӧ��ƽ�ⳣ��K360��400��ʱ��Ӧ��ƽ�ⳣ��K400֮��Ĺ�ϵ��K360_________K400��(����������=����������)��
��һ���¶�������![]() ������HCl��ƽ��ת����_______(��������������С������������)��ԭ��Ϊ___________________��
������HCl��ƽ��ת����_______(��������������С������������)��ԭ��Ϊ___________________��
���𰸡�2HCl(g)+![]() O2(g)=H2O(g)+Cl2(g) H=H1+H2+H3
O2(g)=H2O(g)+Cl2(g) H=H1+H2+H3 ![]() c1V1��10-3 0.56
c1V1��10-3 0.56 ![]() �� �� ��С ����
�� �� ��С ����![]() ����N2��������HCl(g)��O2(g)��H2O(g)��Cl2(g)��Ũ�Ⱦ�ʹ��Ӧ��ϵ��Ũ����Q��K��ƽ�������ƶ�����(HCl)��С
����N2��������HCl(g)��O2(g)��H2O(g)��Cl2(g)��Ũ�Ⱦ�ʹ��Ӧ��ϵ��Ũ����Q��K��ƽ�������ƶ�����(HCl)��С
��������
(1)���ݸ�˹���ɼ����ܷ�Ӧ�ʱ䣻
(2)����![]() �ĵ�λ��֪HCl��������ָ��λʱ����������HCl�����ʵ���������
�ĵ�λ��֪HCl��������ָ��λʱ����������HCl�����ʵ���������![]() �ĵ�����1min����1g������HCl�����ʵ������ݴ˷�������
�ĵ�����1min����1g������HCl�����ʵ������ݴ˷�������
(3)��ͼ��֪��![]() һ��ʱ���¶�Խ��HCl��ת����ԽС����������ӦΪ���ȷ�Ӧ��
һ��ʱ���¶�Խ��HCl��ת����ԽС����������ӦΪ���ȷ�Ӧ��![]() ����Ҳ���ǵ�λʱ����ͨ����λ�����Ĵ�����HCl��O2�������١�
����Ҳ���ǵ�λʱ����ͨ����λ�����Ĵ�����HCl��O2�������١�
(1)���ݸ�˹���ɣ����ܻ�ѧ��Ӧ��һ����ɻ��Ƿּ�����ɣ��䷴Ӧ�ȶ�����ͬ�ģ���ͼ1��֪�Ȼ�ѧ����ʽΪ2HCl(g)+![]() O2(g)=H2O(g)+Cl2(g)H=H1+H2+H3��
O2(g)=H2O(g)+Cl2(g)H=H1+H2+H3��
(2)�ٸ����������ӷ���ʽ2S2O32-+I2=S4O![]() +2l����Cl2����I�������ӷ���ʽΪCl2+2I��=2Cl��+I2�����Եõ����¹�ϵʽ��2S2O32-��I2��Cl2��n(Cl2)=
+2l����Cl2����I�������ӷ���ʽΪCl2+2I��=2Cl��+I2�����Եõ����¹�ϵʽ��2S2O32-��I2��Cl2��n(Cl2)=![]() n(S2O32-)=
n(S2O32-)=![]() c1V1��10-3mol��
c1V1��10-3mol��
�ڵ�![]() =50g��min��mol-1ʱ��ÿ��������1g������HClΪ
=50g��min��mol-1ʱ��ÿ��������1g������HClΪ![]() mol��
mol��![]() =4������ÿ��������1g������O2Ϊ
=4������ÿ��������1g������O2Ϊ![]() mol������������ʵ���Ϊ
mol������������ʵ���Ϊ![]() �����Ϊ
�����Ϊ![]() =0.56L��
=0.56L��
��![]() =200g��min��mol-1ʱ��(HCl)Ϊ33.3%����v(HCl)=
=200g��min��mol-1ʱ��(HCl)Ϊ33.3%����v(HCl)=![]() ��33.3%molg-1min-1�����ݻ�ѧ����ʽHCl��O2��Ӧ�ı���Ϊ4��1��v(O2)=
��33.3%molg-1min-1�����ݻ�ѧ����ʽHCl��O2��Ӧ�ı���Ϊ4��1��v(O2)=![]() v(HCl)=
v(HCl)=![]() ��
��![]() ��33.3%molg-1min-1=
��33.3%molg-1min-1=![]() molg-1min-1��
molg-1min-1��
�ܸ��ݵڢ���ļ�����̿�֪����![]() ��ͬʱ��HCl��ת����Խ��Ӧ����Խ��ͼ��֪410�桢
��ͬʱ��HCl��ת����Խ��Ӧ����Խ��ͼ��֪410�桢![]() =3�������HCl��ת���ʽϴ�����vI��v����
=3�������HCl��ת���ʽϴ�����vI��v����
(3)����ͼ��֪����![]() ��ͬʱ���¶�Խ��HClƽ��ת����ԽС��˵���÷�Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���Kֵ��С����K360��K400��
��ͬʱ���¶�Խ��HClƽ��ת����ԽС��˵���÷�Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���Kֵ��С����K360��K400��
��һ���¶�������![]() ����ͨ�������Ļ��������HCl��O2����С��N2��������൱�ڷ�Ӧ����������Ũ��ͬʱ��С��ͬ����������Ũ����Qc=
����ͨ�������Ļ��������HCl��O2����С��N2��������൱�ڷ�Ӧ����������Ũ��ͬʱ��С��ͬ����������Ũ����Qc=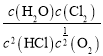 ����������Ũ�ȱ�С��ͬ����ʱ����ʹQc����K����ʱƽ�������ƶ���HCl��ƽ��ת���ʼ�С��
����������Ũ�ȱ�С��ͬ����ʱ����ʹQc����K����ʱƽ�������ƶ���HCl��ƽ��ת���ʼ�С��

����Ŀ�����ڿ��淴ӦN2��g��+3H2��g��![]() 2NH3��g����H��0�������о�Ŀ�ĺ�ʾ��ͼ�������
2NH3��g����H��0�������о�Ŀ�ĺ�ʾ��ͼ�������
A | B | C | D | |
�о�Ŀ�� | ѹǿ�Է�Ӧ��Ӱ��P2��P1 | �¶ȶԷ�Ӧ��Ӱ�� | ƽ����ϵ���ӵ����Է�Ӧ��Ӱ�� | �����Է�Ӧ��Ӱ�� |
ͼʾ |
|
|
|
|
A. AB. BC. CD. D
����Ŀ��ij�л���Ľṹ��ʽ���£�
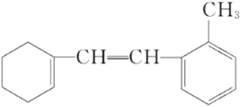
��1�������ʱ�����һ�ȴ�����____________________�֣�
��2��1 mol�����ʺ���ˮ��ϣ�����Br2�����ʵ���Ϊ____________________mol��
��3��1 mol�����ʺ�H2�ӳ������H2____________________mol��
��4������˵������ȷ����____________________��
A�������ʿɷ����ӳɡ�ȡ���������ȷ�Ӧ |
B��������������ˮ |
C����������ʹ��ˮ��ɫ |
D����������ʹ����KMnO4��Һ��ɫ |
E.����ʽ��C15H18