��Ŀ����
����Ŀ����ͼ��ʾ��װ���У��׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH��2K2CO3+6H2O���Իش��������⣺
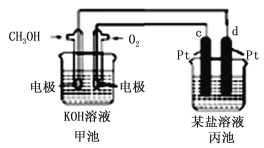
(1)ͼ�м׳�ͨ��O2���ĵ缫��ӦʽΪ____��ͨ��CH3OH�缫�ĵ缫��ӦʽΪ______ ��
(2)��������Ϊ���͵�MgCl2��Һ�������з�Ӧ�����ӷ���ʽΪ__________��
(3)��c����Pt�缫��ΪFe����ҺΪ����ʳ��ˮ����������Ҫ��Ӧ�����ӷ���ʽΪ___��
(4)������װ��1L 0.2 mol��L��1 CuSO4��Һ���տ�ʼʱ���缫c�ĵ缫��ӦʽΪ_______�����һ��ʱ�����������Һ�м���0.2mol��ʽ̼��ͭ��ʹ���ػָ���ԭ����״̬�����·��ת�Ƶ��ӵ����ʵ���Ϊ___________��
���𰸡�3O2��6H2O+12e��=12OH�� 2CH3OH��16OH����12e����2CO32����12H2O Mg2����2Cl����2H2O![]() Mg(OH)2����H2����Cl2�� Fe��2H2O
Mg(OH)2����H2����Cl2�� Fe��2H2O![]() Fe(OH)2����H2�� 4OH����4e����2H2O��O2����2H2O��4e����4H����O2�� 0.6 mol
Fe(OH)2����H2�� 4OH����4e����2H2O��O2����2H2O��4e����4H����O2�� 0.6 mol
��������
(1)�׳�Ϊȼ�ϵ�أ��ҳ�Ϊ���أ��ݴ˷�����
��2����ⱥ�͵��Ȼ�þ��Һ����������������ʧ���ӱ������������������ӵõ��ӱ���ԭ��
��3�����缫���Դ�������������������ǻ��Ե缫���缫����ʧ���ӱ������������������ӵõ��ӱ���ԭ��
��4���������ͭ��Һ����Һ�����ԣ���������Һ�м����ʽ̼��ͭ�ָܻ�ԭ��Һ����ʽ̼��ͭ�����ᷴӦ��������ͭ��ˮ�Ͷ�����̼����Һ�������ӵ�����ͭ�����������ӣ�����ʵ���ϵ������ͭ��Һ�������Σ�
��һ��2CuSO4+2H2O![]() 2Cu��+O2��+2H2SO4��
2Cu��+O2��+2H2SO4��
�ڶ��Σ�2H2O![]() 2H2��+O2��
2H2��+O2��
����ʽ̼��ͭ��ѧʽ�ı�Ϊ2CuOH2OCO2�����Լ���0.1molCu2��OH��2CO3���൱�ڼ���0.2molCuO��0.1molˮ���ݴ˷�����
��1���׳���ȼ�ϵ�أ�ͨ��������Ϊ��Դ��������������ԭ��Ӧ��������Ǽ���Һ�����Ե缫��Ӧʽ�ǣ�3O2��6H2O+12e��=12OH�����״��ǿ�ȼ�����ԭ����ʧ���ӱ����������Ե缫��Ӧʽ�ǣ�2CH3OH��16OH����12e����2CO32����12H2O��
�ʴ�Ϊ��3O2��6H2O+12e��=12OH����2CH3OH��16OH����12e����2CO32����12H2O��
��2����ⱥ�͵��Ȼ�þ��Һ����������������ʧ���ӱ������������������ӵõ��ӱ���ԭ�������ܷ�Ӧʽ�ǣ�Mg2����2Cl����2H2O![]() Mg(OH)2����H2����Cl2����
Mg(OH)2����H2����Cl2����
�ʴ�Ϊ��Mg2����2Cl����2H2O![]() Mg(OH)2����H2����Cl2��
Mg(OH)2����H2����Cl2��
��3�����缫���Դ�������������������ǻ��Ե缫���缫����ʧ���ӱ������������������ӵõ��ӱ���ԭ������������ˮ�ĵ��룬ʹ����������Ũ�����ʱ��صĵ��뷽��ʽ�ǣ�Fe��2H2O![]() Fe(OH)2����H2����
Fe(OH)2����H2����
�ʴ�Ϊ��Fe��2H2O![]() Fe(OH)2����H2��
Fe(OH)2����H2��
��4���������ͭ��Һ��������ˮ���������������ʧȥ���ӱ����������缫c�ĵ缫��ӦʽΪ4OH����4e����2H2O��O2����2H2O��4e����4H����O2����
��һ�Σ�����ͭԭ���غ�֪���������ͭ��Һ����n��Cu��=n��CuO��=0.2mol��ת�Ƶ��ӵ����ʵ���=0.2mol��2=0.4mol��
�ڶ��Σ��������0.1molˮת�Ƶ��ӵ����ʵ���=0.1mol��2=0.2mol��
���Խ�����й�ת�Ƶĵ�����Ϊ0.4mol+0.2mol=0.6mol��
p>�ʴ�Ϊ��4OH����4e����2H2O��O2����2H2O��4e����4H����O2����0.6mol��
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д�