��Ŀ����
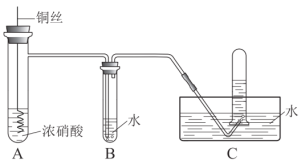
����Ŀ��A��B��D��E��F����Ҫ���л�����ԭ�ϡ���A�IJ�����һ������ʯ�ͻ�����չˮƽ�ı�־��A����Է�������Ϊ28��B������ȼ�Ϻ��ܼ���FΪ����ζ����״Һ�塣����֮���ת����ϵ����ͼ��

��1��A�Ľṹ��ʽ��______����Ӧ������______���Ӧ���ͣ���
��2��B��������______��
��3����Ӧ�ڵĻ�ѧ����ʽ��______��
��4����Ӧ�ܵĻ�ѧ����ʽ��______��
��5������˵������ȷ����______������ţ���
a��A��B�����ܱ����Ը��������Һ����
b���ñ���Na2CO3��Һ�ܳ�ȥF�л��е�����B��E
c�������п�����E��ȥˮ���е�ˮ��
���𰸡�CH2=CH2 �ӳɷ�Ӧ �ǻ� 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3CH2OH+CH3COOH
2CH3CHO+2H2O CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O a
CH3COOCH2CH3+H2O a
��������
A������������һ������ʯ�ͻ�����չˮƽ�ı�־����Է�������Ϊ28����A����ϩ����Ӧ��Ϊ��ϩ��ˮ�����ӳɷ�Ӧ�õ�BΪCH3CH2OH����Ӧ����CH3CH2OH����������Ӧ�õ�DΪCH3CHO����Ӧ����D�������õ�EΪCH3COOH����Ӧ�����Ҵ��������������Ӧ���õ�FΪCH3COOCH2CH3��
�ɷ�����֪��AΪCH2=CH2��BΪCH3CH2OH��DΪCH3CHO��EΪCH3COOH��FΪCH3COOCH2CH3��
��1��A����ϩ���ṹ��ʽ��CH2=CH2����Ӧ������ϩ��ˮ�ļӳɷ�Ӧ���ʴ�Ϊ��CH2=CH2���ӳɷ�Ӧ��
��2��BΪCH3CH2OH�����������ǻ����ʴ�Ϊ���ǻ���
��3����Ӧ�����Ҵ�������������ȩ�ķ�Ӧ����ѧ����ʽ�ǣ�2CH3CH2OH+O2![]() 2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��4����Ӧ�����Ҵ��������������Ӧ����ѧ����ʽ�ǣ�CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��5��a��A����ϩ��B���Ҵ������ܱ����Ը��������Һ������a����
b�����������л��е������Ҵ������ᣬ���ñ���Na2CO3��Һ�ܳ�ȥ���Ҵ���Һˮ�㣬��������Na2CO3��Ӧ��b��ȷ��
c�� CH3COOHҲ�ƴ��ᣬ�������ԣ�����ˮ���е�������̼��ơ�������þ�ȷ�Ӧ���������п����ڳ�ȥˮ���е�ˮ����c��ȷ��
��ѡa���ʴ�Ϊa��

����Ŀ��4���������������Ԫ�ص����λ�������Ԫ��x��ԭ�Ӻ����������m��2����y��������������ԣ�![]() ��Ϊ��±�أ�������±�����ƣ�����˵������ȷ���ǣ� ��
��Ϊ��±�أ�������±�����ƣ�����˵������ȷ���ǣ� ��
M | N | ||
X | Y |
A.Xλ��������IIA���䵥�ʿɲ��õ������![]() �Ʊ�
�Ʊ�
B.Ԫ������������ˮ�����У�������ǿ����![]()
C.�������![]() �ĵ���ʽΪ
�ĵ���ʽΪ![]()
D.![]() ��������������Һ��Ӧ�Ļ�ѧ����ʽΪ
��������������Һ��Ӧ�Ļ�ѧ����ʽΪ![]()