��Ŀ����
����Ŀ�����Ŵ�����Ⱦ���������أ�����������ʮ�������ڼ䣬����������(SO2)�ŷ�������8%����������(NOx)�ŷ�������10%��������̼(CO2)���ŷ���ҲҪ������١�
(1)�ں��£��ݻ�Ϊ1 L�����У�����Է�������ת�����䷴Ӧ���̺�������ϵ��ͼ1��ʾ(��֪:2SO2(g)��O2(g) ![]() 2SO3(g) ��H����196.6 kJ��mol��1)����ش���������:
2SO3(g) ��H����196.6 kJ��mol��1)����ش���������:
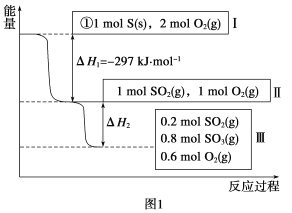
��д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ:________��
����H2��__________kJ��mol��1��
������ͬ�����£�����1 mol SO3��0.5 mol��O2����ﵽƽ��ʱSO3��ת����Ϊ___________����ʱ�÷�Ӧ________(�����ų�������������)________kJ��������
(2)�й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%��50%��
��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2��һ�������·�Ӧ:CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1�����CO2��CH3OH(g)Ũ����ʱ��仯��ͼ2��ʾ����3 min��9 min��v(H2)��________mol��L��1��min��1��
CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1�����CO2��CH3OH(g)Ũ����ʱ��仯��ͼ2��ʾ����3 min��9 min��v(H2)��________mol��L��1��min��1��
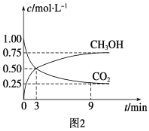
����˵��������Ӧ�ﵽƽ��״̬����________(����)��
A.��Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1(��ͼ�н����)
B.���������ܶȲ���ʱ��ı仯���仯
C.��λʱ��������3 mol H2��ͬʱ����1 mol H2O
D.CO2����������ڻ�������б��ֲ���
��Ϊ�˼ӿ컯ѧ��Ӧ������ʹ��ϵ����������ʵ������٣�������������ʱ���ɲ�ȡ�Ĵ�ʩ��________(����)��
A.�����¶� B.��С������� C.�ٳ���CO2���� D.ʹ�ú��ʵĴ���
(3)��ҵ�ϣ�CH3OHҲ����CO��H2�ϳɡ��ο��ϳɷ�ӦCO(g)��2H2(g) ![]() CH3OH(g)��ƽ�ⳣ��������˵����ȷ����________��
CH3OH(g)��ƽ�ⳣ��������˵����ȷ����________��
�¶�/�� | 0 | 100 | 200 | 300 | 400 |
ƽ�ⳣ�� | 667 | 13 | 1.9��10��2 | 2.4��10��4 | 1��10��5 |
A.�÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ
B.�÷�Ӧ�ڵ����²����Է����У������¿��Է����У�˵���÷�Ӧ��S<0
C.��T ��ʱ��1 L�ܱ������У�Ͷ��0.1 mol CO��0.2 mol H2���ﵽƽ��ʱ��COת����Ϊ50%�����ʱ��ƽ�ⳣ��Ϊ100
D.��ҵ�ϲ����Ըߵ�ѹǿ(5 MPa)��250 ��������Ϊ�������£�ԭ����ת�������
���𰸡�
(1)��S(s)��O2(g) = SO2(g) ��H����297 kJ��mol��1 ��
����78.64����20%�����գ�19.66 ��
(2)��0.125����D����B��
(3)AC��
��������
���������(1)��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ���������ų������������ԣ�������ʵ���Ϊ1mol����ͼ1��֪1molS(s)��ȫȼ�շų�������Ϊ297KJ�����ԣ����ȼ���ȵ��Ȼ�ѧ����ʽS(s)+O2(g)�TSO2(g)��H=-297 KJmol-1���ʴ�Ϊ��S(s)+O2(g)�TSO2(g)��H=-297 KJmol-1��
������ͼ1��֪���μӷ�Ӧ��n(SO2)=1mol-0.2mol=0.8mol�������Ȼ�ѧ����ʽ��2SO2(g)+O2(g)2SO3(g)��H=-196.6KJmol- 1����֪����H2=0.4��H=0.4��(=-196.6KJmol- 1)=-78.64KJmol-1���ʴ�Ϊ��-78.64KJmol-1��
������ͬ�����£�����1 mol SO3��0.5 mol��O2���൱�ڳ���1 mol SO2��1molO2������ͼ����1 mol SO2��1molO2��ƽ��ʱSO2��ת����Ϊ![]() ��100%=80%����ﵽƽ��ʱSO3��ת����Ϊ20%���ֽ��SO3Ϊ0.2mol����ʱ�÷�Ӧ���յ�����Ϊ196.6��
��100%=80%����ﵽƽ��ʱSO3��ת����Ϊ20%���ֽ��SO3Ϊ0.2mol����ʱ�÷�Ӧ���յ�����Ϊ196.6��![]() =19.66 KJ���ʴ�Ϊ��20%�����գ�19.66 ��
=19.66 KJ���ʴ�Ϊ��20%�����գ�19.66 ��
(2)��v(CO2)=![]() =
=![]() =0.417mol/Lmin����v(H2)=3v(CO2)=3��0.417mol/Lmin=0.125mol/(Lmin)-1���ʴ�Ϊ��0.125��
=0.417mol/Lmin����v(H2)=3v(CO2)=3��0.417mol/Lmin=0.125mol/(Lmin)-1���ʴ�Ϊ��0.125��
��A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1ʱ��û�дﵽƽ��״̬����A����B��������������������䣬������������䣬�������Ƿ�ﵽƽ��״̬��������ܶȶ����䣬��B����C����ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ������Ƿ�ﵽƽ��״̬�������ڵ�λʱ����ÿ����3molH2��ͬʱ����1molH2O����C����D��CO2����������ڻ�������б��ֲ��䣬˵���ﵽƽ��״̬����D��ȷ����ѡD��
��A.�����¶ȣ���Ӧ���ʼӿ죬ƽ�������ƶ�����������ʵ���������B.��С�����������Ӧ���ʼӿ죬ƽ�������ƶ�����������ʵ�����С����ȷ��C.�ٳ���CO2���壬��Ӧ���ʼӿ죬ƽ�������ƶ�����������ʵ���������D.ʹ�ú��ʵĴ�������Ӧ���ʼӿ죬ƽ�ⲻ�ƶ�����������ʵ������䣬����ѡB��
(3)A������ƽ�ⳣ�����¶ȱ仯��ƽ���ƶ�ԭ�������жϣ����¶�����ƽ�ⳣ����С������ӦΪ���ȷ�Ӧ��H��0����A��ȷ��B���÷�Ӧ���ڷ��ȷ�Ӧ����H��0���ڵ����²����Է����У������¿��Է����У�������G=��H-T��S��0��˵���÷�Ӧ��S��0����B����C�����ƽ������ʽ��ʽ���㣬ƽ�ⳣ������������ƽ��Ũ���ݴη��˻����Է�Ӧ��ƽ��Ũ���ݴη��˻���
CO(g)+2H2(g)CH3OH(g)
��ʼ��(mol/L) 0.1 0.2 0
�仯��(mol/L) 0.1��50% 0.1 0.05
ƽ����(mol/L) 0.05 0.1 0.05
ƽ�ⳣ��K=![]() =100����C��ȷ��D������ƽ��������У��������Ǵ������������ԭ������ת���ʸߣ���D����ѡAC��
=100����C��ȷ��D������ƽ��������У��������Ǵ������������ԭ������ת���ʸߣ���D����ѡAC��

����Ŀ��һ�������£���2L�����ܱ������г���1mol PCl5��������Ӧ��PCl5(g)![]() PCl3(g)+Cl2(g)
PCl3(g)+Cl2(g)
��Ӧ�����вⶨ�IJ������ݼ��±�����Ӧ�������������䣩:
t/s | 0 | 50 | 150 | 250 | 350 | 450 | |
n(PCl3)/mol | 0 | 0.16 | 0.19 | 0.2 | 0.2 | x | |
��ش���������:
(1) x ��ֵ��________��
(2) 0-50s �ڣ���PCl3��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ������________��
(3)250s �Ժ�Cl2�����ʵ������ٸı��ԭ����_______________��
(4) 250s ʱ��������Cl2�����ʵ�����_____��PCl5�����ʵ�����______��PCl5��ת������______��

