��Ŀ����
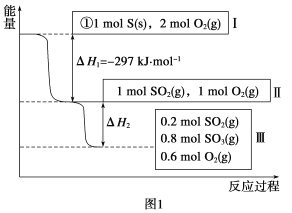
����Ŀ��һ�������£���2L�����ܱ������г���1mol PCl5��������Ӧ��PCl5(g)![]() PCl3(g)+Cl2(g)
PCl3(g)+Cl2(g)
��Ӧ�����вⶨ�IJ������ݼ��±�����Ӧ�������������䣩:
t/s | 0 | 50 | 150 | 250 | 350 | 450 | |
n(PCl3)/mol | 0 | 0.16 | 0.19 | 0.2 | 0.2 | x | |
��ش���������:
(1) x ��ֵ��________��
(2) 0-50s �ڣ���PCl3��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ������________��
(3)250s �Ժ�Cl2�����ʵ������ٸı��ԭ����_______________��
(4) 250s ʱ��������Cl2�����ʵ�����_____��PCl5�����ʵ�����______��PCl5��ת������______��
���𰸡� 0.2 0.0016mol/(L��s) 250s �ﵽƽ�⣬���淴Ӧ������ȣ��������䣬����ֵ����ʵ��������ٱ仯 0. 2mol 0. 8mol 20%
�����������������������Ҫ���黯ѧƽ�⡣
(1)150sʱ�ﵽƽ��״̬��x ��ֵ��0.2��
(2) 0-50s �ڣ���PCl3��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ������0.16/(2��50)mol/(L��s)=0.0016mol/(L��s)��
(3)250s �Ժ�Cl2�����ʵ������ٸı��ԭ����250s �ﵽƽ�⣬���淴Ӧ������ȣ��������䣬����ֵ����ʵ��������ٱ仯��
(4) 250s ʱ��������Cl2�����ʵ�������PCl3�����ʵ�����0.2mol��PCl5�����ʵ�����(1-0.2)mol=0.8mol��PCl5��ת������20%��

����Ŀ��
��1��д��������ˮ��Һ�еĵ��뷽��ʽ ����ij�¶��£�CH3COOH(aq)��NaOH(aq)��Ӧ����H=" -" 46.8kJ��mol-1��HCl(aq)��NaOH(aq)��Ӧ����H=" -" 55.6 kJ��mol-1����CH3COOH��ˮ��Һ�е������H= kJ��mol-1��
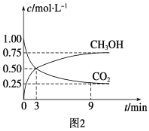
��2��ij�¶��£�ʵ����0.1mol��L-1��������ԼΪ1.5%������¶���0.1mol��L-1CH3COOH�ĵ���ƽ�ⳣ��K=________���г�����ʽ����֪�����![]() ��
��
��3����������ѧ���о�����������ϩ������Ϊԭ�ϡ��Ӷ����������ϳ������������¹��գ����������Ҵ�����ȩ���м��壬ʹ��Ʒ�ɱ����ͣ��������Ծ������ơ���ϳɵĻ�����Ӧ���£�
![]()
����������˵����ϩ������ϳ����������ķ�Ӧ�Ѵﻯѧƽ����� ��
A����ϩ�����ᡢ����������Ũ����ͬ |
B�������ϳɷ�Ӧ�����������ֽⷴӦ��������� |
C����ϩ�Ͽ�1mol̼̼˫����ͬʱ����ǡ������1mol |
D����ϵ����ϩ�İٷֺ���һ�� |
��4����n(��ϩ)��n(����)���ϱ�Ϊ1�������£�ij�о�С���ڲ�ͬѹǿ�½���������ͬʱ������������IJ������¶ȵı仯�IJⶨʵ�飬ʵ������ͼ��ʾ���ش��������⣺
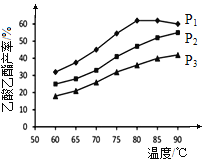
�� �¶���60��80����Χ�ڣ���ϩ�����������ϳɷ�Ӧ�����ɴ�С��˳���� [��![]() (P1)��
(P1)��![]() (P2)��
(P2)��![]() (P3)�ֱ��ʾ��ͬѹǿ�µķ�Ӧ����]��������ԭ��Ϊ ��
(P3)�ֱ��ʾ��ͬѹǿ�µķ�Ӧ����]��������ԭ��Ϊ ��
��ѹǿΪP1MPa���¶�60��ʱ�������������IJ���Ϊ30�G�����ʱ��ϩ��ת����Ϊ ��
����ѹǿΪP1MPa���¶ȳ���80��ʱ���������������½���ԭ�������_________��
�����ݲⶨʵ���������������˵����������� ��������ʵ�ѹǿ���¶ȣ���Ϊ������������ĺϳ����ʺͲ��ʣ����Բ�ȡ�Ĵ�ʩ�� ����д��һ������