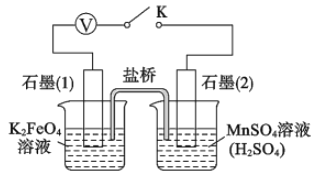
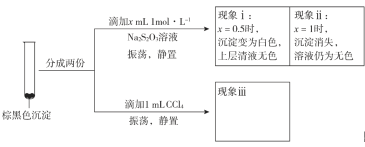
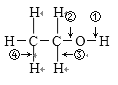
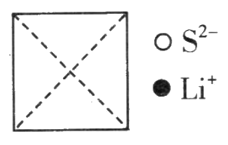��Ŀ����
����Ŀ�����ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol/L��HCl����Һ�����к͵ζ����ü�����ָʾ������ ��ش��������⣺
(1)�ζ�ǰ������Һ����ȡ����NaOH��Һ�� _____�У� ���������ƣ���������2~3��ָʾ����
(2)ʢװ���������������Ϊ _____ ��
(3)����жϵζ��յ㣨��д����ɫ�仯�� __________ ��
ijѧ����������ƽ��ʵ�飬���ݼ�¼���£�
(4)ѡȡ�����������ݣ��������NaOH��Һ�����ʵ���Ũ��Ϊ ___________����������λ��Ч���֣�
ʵ����� | ����NaOH��Һ�����/ mL | 0.1000mol.L-1HCl��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.00 | 26.29 |
2 | 25.00 | 1.00 | 31.00 |
3 | 25.00 | 1.00 | 27.31 |
(5)������Щ������ʹ�ⶨ���ƫ��____________��
a.��Ӧ����������ˮϴ����δ�ô���Һ��ϴ
b.�ζ�ǰ��ƽ�Ӷ������ζ������Ӷ���
c.�ζ�ǰ�����������ݣ��ζ������������
���𰸡���ƿ ��ʽ�ζ��� ��Һ�ɻ�ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ 0.1052mol/L c
��������
(1)�ζ�������Һ�ڵζ����У�����Һʢ����ƿ�У�
(2)������Һ�������ʽ�ζ����У�
(3)���ݵζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���ұ��ְ���Ӳ���ɫ��
(4)�ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V(����)�����Ÿ��������NaOH��Ӧ���c(NaOH)��
(5)����c(����)=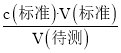 ��������������V(��)��Ӱ�죬�Դ������
��������������V(��)��Ӱ�죬�Դ������
(1)NaOHΪ����Һ���ü�ʽ�ζ���ȡ����NaOH��Һ����ƿ�У�
(2)ʢװ���������������Ϊ��ʽ�ζ��ܣ�
(3)����Һ���������ƣ���ƿ��ʢ�е�����������Һ�е�����ȣ�����ָʾ���ı�ɫ��Χ��֪����Һ����ɫ�ǻ�ɫ��������Һ��pH��С�����ε���Һ��pHС��4.4ʱ����Һ��ɫ�ɻ�ɫ��ɳ�ɫ���Ұ���Ӳ���ɫ���ε��������ʵζ��յ������Ϊ����Һ�ɻ�ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��
(4)�������ݵ���Ч�ԣ���ȥ��2�����ݣ���1��3��ƽ������V(����)=![]() =26.30mL����HCl+NaOH�TNaCl+H2O��֪��0.0263L��0.1000mol/L=0.025L��c(NaOH)�����c(NaOH)=0.1052mol/L��
=26.30mL����HCl+NaOH�TNaCl+H2O��֪��0.0263L��0.1000mol/L=0.025L��c(NaOH)�����c(NaOH)=0.1052mol/L��
(5) a.��Ӧ����������ˮϴ����δ�ô���Һ��ϴ�����ڴ������Һ�����ʵ����ʵ������䣬��˶�������Һ��Ũ����Ӱ�죬a���������⣻
b.�ζ�ǰ��ƽ�Ӷ������ζ������Ӷ����������ı�����Һ���ƫ����c(����)=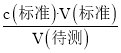 ��֪��V(��)ƫ����ᵼ��c(����)ƫ��b���������⣻
��֪��V(��)ƫ����ᵼ��c(����)ƫ��b���������⣻
c.�ζ�ǰ�����������ݣ��ζ�����������ݣ������ı�����Һ���ƫ�ͣ�����c(����)=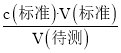 ��֪��V(��)ƫ�ͣ���ᵼ��c(����)ƫ�ͣ�c�������⣻
��֪��V(��)ƫ�ͣ���ᵼ��c(����)ƫ�ͣ�c�������⣻
�ʺ���ѡ����c��

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�