��Ŀ����
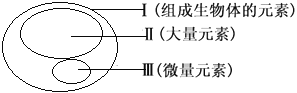
����Ŀ��X��Y��Z��WΪ���ֶ���������Ԫ�أ�X��Y�����ڱ��е����λ����ͼ��ʾ��Xn��Yn+��Z+������ͬ�ĵ��Ӳ�ṹ��W�����ڲ������������������֮�͵��ڴ���������������˵����ȷ����

A. ԭ�Ӱ뾶��r(X)<r(Y)<r(Z)<r(W)
B. X�γɵ���������������Ϊ4��
C. ����������Ӧˮ����ļ��ԣ�Z<Y
D. Y��Z��W��Ӧ������������ˮ����֮���ܹ��������Ӧ
���𰸡�D
��������W�����ڲ������������������֮�͵��ڴ�����������W��3�����Ӳ㣬����������Ϊ6��WΪSԪ�أ�Xn��Y n+���е�ɵľ���ֵ��ͬ�����һ�����壬���ֱ���N3��Al3+��ZΪNaԪ�ء�ԭ�Ӱ뾶r(Na)>r(Al)>r(S)>r(N)��A����Ԫ���γɵ���������N2O��NO��N2O3��NO2��N2O4��N2O5����B����Na��Al ����������Ӧˮ����ΪNaOH��Al(OH)3�����ԣ�NaOH>Al(OH)3��C��������Ԫ�ض�Ӧ������������ˮ����ΪNaOH��Al(OH)3��H2SO4������Al(OH)3Ϊ���Ի������ܺ���Ӧ��D��ȷ��

����Ŀ�����ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol/L��HCl����Һ�����к͵ζ����ü�����ָʾ������ ��ش��������⣺
(1)�ζ�ǰ������Һ����ȡ����NaOH��Һ�� _____�У� ���������ƣ���������2~3��ָʾ����
(2)ʢװ���������������Ϊ _____ ��
(3)����жϵζ��յ㣨��д����ɫ�仯�� __________ ��
ijѧ����������ƽ��ʵ�飬���ݼ�¼���£�
(4)ѡȡ�����������ݣ��������NaOH��Һ�����ʵ���Ũ��Ϊ ___________����������λ��Ч���֣�
ʵ����� | ����NaOH��Һ�����/ mL | 0.1000mol.L-1HCl��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.00 | 26.29 |
2 | 25.00 | 1.00 | 31.00 |
3 | 25.00 | 1.00 | 27.31 |
(5)������Щ������ʹ�ⶨ���ƫ��____________��
a.��Ӧ����������ˮϴ����δ�ô���Һ��ϴ
b.�ζ�ǰ��ƽ�Ӷ������ζ������Ӷ���
c.�ζ�ǰ�����������ݣ��ζ������������
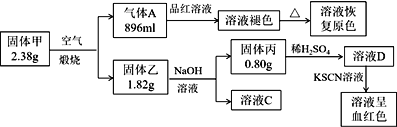
����Ŀ����.Ϊ��̽��һ�ֹ��廯����ף�����3��Ԫ�أ�����ɺ����ʣ���Ʋ��������ʵ�飺(��������Ѿ�����ɱ�״���µ������
��ش�
��1��д��������Ļ�ѧʽ________��
��2��д���γ���ҺC�Ļ�ѧ����ʽ��_____________��
��3��д������Aͨ����ҺD�У�������Ӧ�����ӷ�Ӧ����ʽ__________��
��.��������ѧ�����о����������� H2��CH3COOH Ϊԭ�Ϻϳ��Ҵ�����Ӧ��ͬʱ�ᷢ������Ӧ��
��Ӧ��.CH3COOH(g)+2H2(g)![]() CH3CH2OH(g) +H2O(g) ��H1
CH3CH2OH(g) +H2O(g) ��H1
��Ӧ��. CH3COOH(g)+H2(g)![]() CO(g)+CH4(g)+H2O(g) ��H2��0
CO(g)+CH4(g)+H2O(g) ��H2��0
��֪���Ҵ�ѡ������ת���������������Ҵ��İٷֱȡ���ش�
��1����Ӧ��һ�����������Է����У����H1 ___0����������������������
��2��ijʵ���п��� CH3COOH �� H2 ��ʼͶ�ϱ�Ϊ 1��1.5������ͬѹǿ�£�������ͬ��Ӧʱ��������ʵ�����ݣ�
�¶ȣ�K�� | ���� | �����ת���ʣ�%�� | �Ҵ�ѡ���ԣ�%�� |
573 | �� | 40 | 50 |
573 | �� | 30 | 60 |
673 | �� | 55 | 35 |
673 | �� | 40 | 50 |
�����������CH3COOHת��ΪCH3 CH2OHƽ��ת���ʵĴ�ʩ��______��
A ʹ�ô����� B ʹ�ô�����
C ���ͷ�Ӧ�¶� D Ͷ�ϱȲ��䣬���ӷ�Ӧ���Ũ��
E ����CH3COOH��H2�ij�ʼͶ�ϱ�
��673K�״��������·�Ӧ���Ѵ�ƽ��״̬����������ת����Ϊ50%���Ҵ���ѡ����40%������ʱ�������Ϊ 1.0L��CH3COOH ��ʼ������Ϊ2.0mol����Ӧ���ƽ�ⳣ�� K= _____��
�۱���ʵ�����ݱ���������ͬ�¶��²�ͬ�Ĵ�����CH3COOHת����CH3CH2OH��ѡ������������Ӱ�죬��ԭ����_________________��
��3����ͼ�зֱ�I�ڴ����ʹ������������������Ӧ����-������ʾ��ͼ��_____