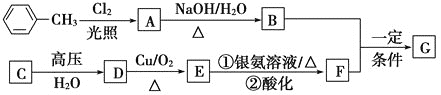
��Ŀ����
����Ŀ��ʵ��С��̽��KI��Cu(NO3)2�ķ�Ӧ������ʵ��һ��
ʵ��һ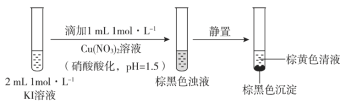
ע����ʵ�����Cu2+�ڴ������µ�ˮ�⡣
��1��ȡ�ػ�ɫ��Һ����������______��Һ���Լ�a������Һ��Ϊ______ɫ��˵��������I2��
��2��̽������I2��ԭ��
�ټ�ͬѧ��������ʵ�飺��2mL1mol��L-1KI��Һ�м���1mL______��Һ�������ữ��pH=1.5�����ټ��������Լ�a���۲쵽�루1����ͬ������ͬѧ�ɴ˵ó����ۣ�ʵ��һ������I2��ԭ�������������£�![]() ������I����
������I����
����ͬѧ��Ϊ���ɼ�ʵ�黹���ܵó���Ӧ���ۡ����������Ǹ�ʵ��û���ų�____________����I���Ŀ����ԡ�
����Ҫȷ֤ʵ��һ����![]() ������I����Ӧ��ʵ��һ�Ļ����Ͻ��м���______��ʵ�顣
������I����Ӧ��ʵ��һ�Ļ����Ͻ��м���______��ʵ�顣
��3��̽���غ�ɫ��������ɡ�
�ٲ������ϵ�֪��CuIΪ������ˮ�İ�ɫ���塣���Ƕ��غ�ɫ���������������ּ��裺
a��CuI����I2b��_________����I2��Ϊ֤�����������Ƿ������ȡ�غ�ɫ��������ʵ�����
ʵ���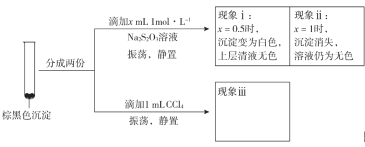
��֪��CuI������CCl4��I2��![]() ==2I����
==2I����![]() ����ɫ����Cu++S2O32-==
����ɫ����Cu++S2O32-==![]() ����ɫ������ʵ����ó����ۣ��غ�ɫ������CuI����I2�γɵġ�
����ɫ������ʵ����ó����ۣ��غ�ɫ������CuI����I2�γɵġ�
������iiiΪ______________��
���û�ѧƽ���ƶ�ԭ�����Ͳ�������ii��ԭ��________________��
���𰸡����� �� 2mol��L1NaNO3����KNO3�� ��Һ�е�Cu2+�������е�O2 NO3-�Ļ�ԭ���NO��NO2�ȣ� Cu �غ�ɫ������ɫ��dz����Һ��Ϊ�Ϻ�ɫ CuI����Һ�д��ڳ����ܽ�ƽ�⣺CuI(s)![]() Cu+(aq)+I-(aq)����������Na2S2O3��Һ��S2O32-��Cu+��Ӧ����Cu(S2O3)23-ʹc(Cu+)��С��ƽ�����ƴӶ�ʹ��ɫ�����ܽ�
Cu+(aq)+I-(aq)����������Na2S2O3��Һ��S2O32-��Cu+��Ӧ����Cu(S2O3)23-ʹc(Cu+)��С��ƽ�����ƴӶ�ʹ��ɫ�����ܽ�
��������
ʵ��һ��KI��Һ�еμ������ữ������ͭ��Һ��Ӧ�õ��غ�ɫ��Һ�����õõ��غ�ɫ�������ػ�ɫ��Һ����1��ȡ�ػ�ɫ��Һ�������������ۡ������ⵥ�ʱ���ɫ��
��2����̽�����������������Һ�е�ǿ�����ԣ����������������ɵⵥ�ʣ�����ȡ�����������Ũ����ͬ����������֤��
����Һ���ܽ��O2��ͭ����Ҳ���������ԣ�������������Ϊ�ⵥ�ʣ�
����Ҫȷ֤ʵ��һ����NO3��������I������Ҫ��֤��������ӱ���ԭ���ɵIJ��һ����������������Ĵ��ڣ�
��3���ٽ�ϼ���a��ͭ���ӻ��ϼ۽���Ϊ+1�ۣ�Ҳ���ܽ�Ϊ0�ۣ�
��I2�������л��ܼ���CuI������CCl4��
��CuI����Һ�д��ڳ����ܽ�ƽ�⣺CuI(s)![]() Cu+(aq)+I-(aq)����������Na2S2O3��Һ��S2O32-��Cu+��Ӧ����Cu(S2O3)23-ʹc(Cu+)��С��
Cu+(aq)+I-(aq)����������Na2S2O3��Һ��S2O32-��Cu+��Ӧ����Cu(S2O3)23-ʹc(Cu+)��С��
��1��KI��Һ�еμ������ữ������ͭ��Һ��Ӧ�õ��غ�ɫ��Һ�����õõ��غ�ɫ�������ػ�ɫ��Һ��ȡ�ػ�ɫ��Һ����������������Һ���Լ�a������Һ��Ϊ��ɫ��֤�������˵ⵥ�ʣ��ʴ�Ϊ�����ۣ� ����
��2��������ʵ����ͭ�����п������������ӣ������Ҫ�ų�ͭ���ӵĸ��ţ���ͬѧ��������ʵ�飺��2mL1mol��L��1KI ��Һ�м���1mL2 mol��L��1 NaNO3����KNO3������Һ�� �����ữ��pH=1.5 �����ټ��������Լ�a���۲쵽�루1����ͬ������ͬѧ�ɴ˵ó����ۣ�ʵ��һ������I2 ��ԭ�������������£�NO3�� ������I����
�ʴ�Ϊ��2 mol��L��1 NaNO3����KNO3����
����ͬѧ��Ϊ���ɼ�ʵ�黹���ܵó���Ӧ���ۡ����������Ǹ�ʵ��û���ų���Һ�е�Cu2������Һ���ܽ��O2����I���Ŀ����ԣ��ʴ�Ϊ����Һ�е�Cu2���������е�O2��
����Ҫȷ֤ʵ��һ����NO3��������I����Ӧ��ʵ��һ�Ļ����Ͻ��м���NO3���Ļ�ԭ���NO��NO2�ȣ���ʵ�飬�ʴ�Ϊ��NO3���Ļ�ԭ���NO��NO2�ȣ���
��3���ٽ�ϼ���a��ͭ���ӻ��ϼ۽���Ϊ+1�ۣ�Ҳ���ܽ�Ϊ0�ۣ���˼���b��Cu����I2���ʴ�Ϊ��Cu��
��I2�������л��ܼ���CuI������CCl4�����ʵ��iii���������غ�ɫ������ɫ��dz�����Ȼ�̼��Һ�仯Ϊ�Ϻ�ɫ���ʴ�Ϊ���غ�ɫ������ɫ��dz����Һ��Ϊ�Ϻ�ɫ��
���û�ѧƽ���ƶ�ԭ�����Ͳ�������ii��ԭ��CuI����Һ�д��ڳ����ܽ�ƽ�⣺CuI(s)![]() Cu+(aq)+I-(aq)����������Na2S2O3��Һ��S2O32-��Cu+��Ӧ����Cu(S2O3)23-ʹc(Cu+)��С��ƽ�����ƴӶ�ʹ��ɫ�����ܽ⣻
Cu+(aq)+I-(aq)����������Na2S2O3��Һ��S2O32-��Cu+��Ӧ����Cu(S2O3)23-ʹc(Cu+)��С��ƽ�����ƴӶ�ʹ��ɫ�����ܽ⣻
�ʴ�Ϊ��CuI����Һ�д��ڳ����ܽ�ƽ�⣺CuI(s)![]() Cu+(aq)+I-(aq)����������Na2S2O3��Һ��S2O32-��Cu+��Ӧ����Cu(S2O3)23-ʹc(Cu+)��С��ƽ�����ƴӶ�ʹ��ɫ�����ܽ⡣
Cu+(aq)+I-(aq)����������Na2S2O3��Һ��S2O32-��Cu+��Ӧ����Cu(S2O3)23-ʹc(Cu+)��С��ƽ�����ƴӶ�ʹ��ɫ�����ܽ⡣

����Ŀ�����ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol/L��HCl����Һ�����к͵ζ����ü�����ָʾ������ ��ش��������⣺
(1)�ζ�ǰ������Һ����ȡ����NaOH��Һ�� _____�У� ���������ƣ���������2~3��ָʾ����
(2)ʢװ���������������Ϊ _____ ��
(3)����жϵζ��յ㣨��д����ɫ�仯�� __________ ��
ijѧ����������ƽ��ʵ�飬���ݼ�¼���£�
(4)ѡȡ�����������ݣ��������NaOH��Һ�����ʵ���Ũ��Ϊ ___________����������λ��Ч���֣�
ʵ����� | ����NaOH��Һ�����/ mL | 0.1000mol.L-1HCl��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.00 | 26.29 |
2 | 25.00 | 1.00 | 31.00 |
3 | 25.00 | 1.00 | 27.31 |
(5)������Щ������ʹ�ⶨ���ƫ��____________��
a.��Ӧ����������ˮϴ����δ�ô���Һ��ϴ
b.�ζ�ǰ��ƽ�Ӷ������ζ������Ӷ���
c.�ζ�ǰ�����������ݣ��ζ������������
����Ŀ��λ��ǰ�����ڵ�6������Ԫ��A��B��C��D��E��Fԭ������������������B��Dͬ���壬 D��Eͬ���ڡ�A��B��C�����ڱ������ڣ�������Ԫ�ص�ԭ������������֮��Ϊ18��F������������������õĽ���Ԫ�ء������ƶϻش��������⣺
(1)A�����ڱ��е�λ��_____��д��A���ʵĵ���ʽ________��
(2)������������������գ�
���Ӱ뾶 | �ǽ����� | ���� | �⻯����ȶ��� |
F+_D2�� | A_B | D������������ˮ����___E������������ˮ���� | C���⻯��__E���⻯�� |
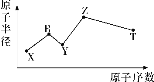
(3)������������ˮ��Һ��1molE-��![]() (x��1��2��3��4)������(KJ)��Դ�С��ͼ��ʾ��
(x��1��2��3��4)������(KJ)��Դ�С��ͼ��ʾ��
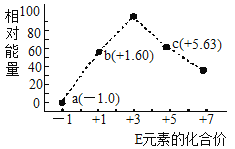
��c���Ӧ������_______(�����ӷ���)��
��b��a+c��Ӧ�����ӷ���ʽΪ________���÷�Ӧ��______��Ӧ(��������������������)��