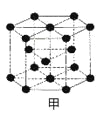
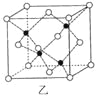
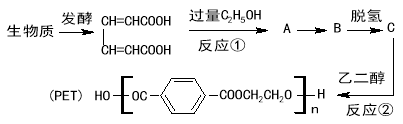
��Ŀ����
����Ŀ����1��������һ��������Դ��ũ����ڴ����Ľոѡ��Ӳݵȷ�������Ǿ������֮��ɲ��������������������������ũ���ƹ㽨�������أ���������Ч������
�ܣ�����Ϊũҵ�����ṩ�����ķ��ϡ���֪�� ��״���µ�112.0L CH4������ȫȼ�գ�����CO2��Һ̬ˮ���ų�4448kJ��������
��д��CH4��ȫȼ�յ��Ȼ�ѧ����ʽΪ ��
�����������Ӧ���ɵ���ˮ��������Ӧ�ų������� 4448kJ�������������������
��2����֪��1 mol H��H����1 molN��N��1 mol N��H���ֱ���Ҫ���յ�����Ϊ436 .4kJ��941.3 kJ��390.5 kJ������1 mol NH3 �ֽ����������͵�����Ҫ ����ų��������ա��� kJ��������
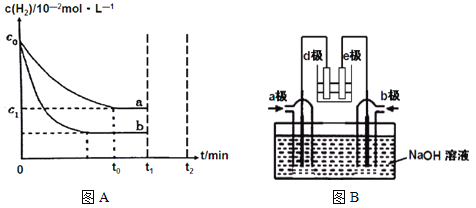
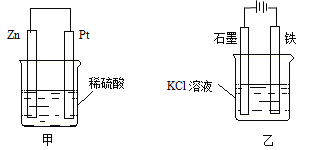
��3���������������о���������ѧ������ܵ��ת���������мס�����װ���У����и����缫��ӦʽΪ ���������缫�ĵ缫��ӦʽΪ �����缫���� ��Ӧ�����������ԭ������
���𰸡���1�������� CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H= -889.6KJmol-1 ��
��2������ 46.25��3��Zn-2e-=Zn2+ 2H++2e-=H2�� ��ԭ
��������
�����������1����ũ���ƹ㽨�������أ���������Ч�������������ܣ�����Ϊũҵ�����ṩ�����ķ��ϡ���״���µ�112.0L�����ʵ���Ϊ5mol CH4������ȫȼ�գ�����CO2��Һ̬ˮ���ų�4448kJ����������1mol������ȫȼ�շų�����4448/5=889.6kJ��
��CH4��ȫȼ�յ��Ȼ�ѧ����ʽΪCH4(g) +2O2(g)=CO2(g)+2H2O(l) ��H= -889.6KJmol-1��
�����������Ӧ���ɵ���ˮ������Һ̬ˮ��Ϊˮ������Ҫ������������Ӧ�ų���������4448kJ��
��2�����ݷ�ӦN2+3H2![]() 2NH3���Լ���Ϣ��1 mol H��H����1 molN��N��1 mol N��H���ֱ���Ҫ���յ�����Ϊ436 .4kJ��941.3 kJ��390.5 kJ����֪����2molNH3�ų�������Ϊ390.5��6-��436 .4��3+941.3��=92.5 kJ������1mol NH3�ֽ����������͵�����Ҫ����92.5/2=46.25kJ��������
2NH3���Լ���Ϣ��1 mol H��H����1 molN��N��1 mol N��H���ֱ���Ҫ���յ�����Ϊ436 .4kJ��941.3 kJ��390.5 kJ����֪����2molNH3�ų�������Ϊ390.5��6-��436 .4��3+941.3��=92.5 kJ������1mol NH3�ֽ����������͵�����Ҫ����92.5/2=46.25kJ��������
��3������п���������缫��ӦʽΪZn-2e-=Zn2+���������缫���������缫��ӦʽΪ2H++2e-=H2�������缫������ԭ��Ӧ��

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
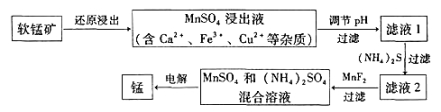
��ѧ����ϵ�д�����Ŀ��ij�����ĵ�������к���ͭ�����Ƚ��������Ϊʵ����Դ�Ļ������ò���Ч��ֹ������Ⱦ,������¹�������:

������ | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 |
��ʼ������pH | 2.3 | 7.6 | 4.4 |
��ȫ������pH | 3.2 | 9.7 | 6.4 |
��1����������H2O2��Ŀ���� ����pH�����м�����Լ������ (�ѧʽ)��ʵ���ҽ��й��˲������õ��IJ��������� �� �� ��
��2�����CuSO4��Һ��ԭ���� ����CuSO4��Һ�м���һ������NaCl��Na2SO3,�������ɰ�ɫ��CuCl����,д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��Fe3+����ˮ�ⷴӦFe3++3H2O![]() Fe(OH)3+3H+,�÷�Ӧ��ƽ�ⳣ������ʽΪ ��
Fe(OH)3+3H+,�÷�Ӧ��ƽ�ⳣ������ʽΪ ��
��4����ȡ���Ʊ���CuCl��Ʒ0.250 0 g����һ������0.5 mol��L-1FeCl3��Һ��,����Ʒ��ȫ�ܽ��,��ˮ20 mL,��0.100 0 mol��L-1��Ce(SO4)2��Һ�ζ�,�����յ�ʱ����Ce(SO4)2��Һ25.00 mL���йصĻ�ѧ��ӦΪFe3++CuCl=Fe2++Cu2++Cl-,Ce4++Fe2+=Fe3++Ce3+�������CuCl��Ʒ���������� ��