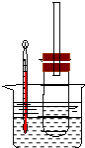
��Ŀ����
5����ҵ�ϳ����ú����ˮ����Na2S2O3•5H2O��ʵ���ҿ�����ͼ��ʾװ�ã���ȥ���ּӳ�������ģ�����ɹ��̣���Cװ����ʢװNa2S��Na2SO3�����Һ��
��ƿC�з�����Ӧ���£�
Na2S+H2O+SO2=Na2SO3+H2S ����
2H2S+SO2=3S��+2H2O ����
S+Na2SO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O ����
��1��������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һע����Һ���߶ȱ��ֲ��䣬������װ�����������ã�
��2��װ��D�������Ƿ�ֹ������װ��E��ΪNaOH��Һ��
��3��Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2S��Na2SO3ǡ����ȫ��Ӧ������ƿC��Na2S��Na2SO3���ʵ���֮��Ϊ2��1��
��4��װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ��c��
a������ˮ b������Na2SO3��Һ
c������NaHSO3��Һ d������NaHCO3��Һ
��5����֪��Ӧ������Խ���������ƿC�з�Ӧ�ﵽ�յ����������Һ����壨�������ʧ����
��6����Ӧ��ֹ����ƿC�е���Һ������Ũ����������Na2S2O3•5H2O�����п��ܺ���Na2SO3��Na2SO4�����ʣ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ�ȡ������Ʒ��������ϡ�����У����ã�ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�BaCl2��Һ�������ְ�ɫ������˵������Na2SO4���ʣ�
��֪Na2S2O3•5H2O�����ֽ⣺S2O32?+2H+=S��+SO2��+H2O
��ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ��
���� ��1������Һ�����һ��ʱ�䲻����������ԣ�
��2��D�ɷ�ֹҺ�嵹����E��ʢ��NaOH��Һ����β��������
��3��C��Na2S��Na2SO3ǡ����ȫ��Ӧ������ձ�C�еķ�Ӧ������
��4���۲�SO2���������ʣ�װ��B�е���Һ�������������ӦҲ�������ն������ݴ˴��⣻
��5������C�з����ķ�Ӧ��֪����ƿC�з�Ӧ�ﵽ�յ㷢���ķ�ӦΪ�����������Ʒ�Ӧ������������ƣ�
��6������Ʒ���Ƿ����Na2SO4���ȼ������ų����ţ��������Ȼ���������������ӣ�
��� �⣺��1��������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һ������Һ���߶ȱ��ֲ��䣬�����������ã�
�ʴ�Ϊ��Һ���߶ȱ��ֲ��䣻
��2��D�����Ϊ�̵��ܣ�Ϊ��ȫƿ����ֹ������װ��E������β����SO2��H2S�����ã���ѡ��NaOH��Һ��
�ʴ�Ϊ����ֹ������NaOH��
��3��C��Na2S��Na2SO3ǡ����ȫ��Ӧ����Na2S��aq��+H2O��l��+SO2��g���TNa2SO3��aq��+H2S��aq������
2H2S��aq��+SO2��g���T3S��s��+2H2O��l������
S��g��+Na2SO3��aq��$\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O3��aq������
��֪������2+����+����3���õ��ܷ�ӦΪ2Na2S��aq��+Na2SO3��aq��+3SO2��g��$\frac{\underline{\;\;��\;\;}}{\;}$3Na2S2O3��aq������C��Na2S��Na2SO3���ʵ���֮��Ϊ2��1��
�ʴ�Ϊ��2��1��
��4���۲�SO2���������ʣ�����ǿ����ȡ����ķ�Ӧ��a�����ɶ�������bd�����ʾ����������Ӧ��ֻ��c�б���NaHSO3��Һ�ʺ���ȡ��������
�ʴ�Ϊ��c��
��5������C�з����ķ�Ӧ��֪����ƿC�з�Ӧ�ﵽ�յ㷢����ӦΪ�����������Ʒ�Ӧ������������ƣ�����Ӧ������Ϊ��Һ����壨�������ʧ����
�ʴ�Ϊ����Һ����壨�������ʧ����
��6������Ʒ���Ƿ����Na2SO4������������ͽ���Ϊȡ������Ʒ��������ϡ�����У����ã�ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�BaCl2��Һ�������ְ�ɫ������˵������Na2SO4���ʣ�
�ʴ�Ϊ��ȡ������Ʒ��������ϡ�����У����ã�ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�BaCl2��Һ�������ְ�ɫ������˵������Na2SO4���ʣ�
���� ���⿼��ʵ�鷽���ķ��������ۣ��漰�����Լ��顢���Ӽ��顢�Բ����ķ������ۡ���ѧ����ȣ�����ʵ�����������֪ʶ�ۺ�Ӧ�������Ŀ��飬��3��Ϊ�״��㣬���������ܷ�Ӧ�ķ�������Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��������NaOH���壬��Һ��c��OH-������ | |
| B�� | ͨ������HCl���壬ƽ�������ƶ� | |
| C�� | ��ˮ����Ӧ��������ƽ�������ƶ� | |
| D�� | ��������NH4Cl���壬ƽ�������ƶ� |
�ٷ�Ӧ���ʵı仯 ��Ũ�ȵı仯 �۸���ְٷֺ����ı仯 ��ƽ����Է��������ı仯
����ɫ�ı仯 ��������ܶȵı仯 ��ת���ʵı仯 ���¶ȵı仯��
| A�� | �٢ڢޢߢ� | B�� | �ڢܢݢޢ� | C�� | �ڢܢݢޢ� | D�� | �ۢܢߢ� |
| A�� | ʯ�;��������ѻ��ȷ����õ������ʾ�Ϊ������ | |
| B�� | ������������֬�������ǡ������ʾ����Է���ˮ�ⷴӦ | |
| C�� | ������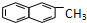 �DZ���ͬϵ�� �DZ���ͬϵ�� | |
| D�� | �ع��ͺͿ����Ϳ�ͨ��������������������Һ���Ⱥ�IJ�ͬ����������� |
| ��֪ | ���� | |
| A | ��Fe����CuSO4��Һ�� Fe+Cu2+=Cu+Fe2+ | ��Na���뵽CuSO4��Һ�� 2Na+Cu2+=Cu+2Na+ |
| B | ����������Һ��ͨ�������̼���� Ca2++2ClO-+CO2+H2O=CaCO3��+2HClO | ����������Һ��ͨ������������� Ca2++2ClO-+SO2+H2O=CaSO3��+2HClO |
| C | �ö��Ե缫���CuSO4��Һ 2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H- | �ö��Ե缫���CuCl2��Һ 2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H- |
| D | ������CaCO3��ĩͶ������������ CaCO3+2H+�TCa2++CO2��+H2O | ������CaCO3��ĩͶ������������Һ�� CaCO3+2H+�TCa2++CO2��+H2O |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���ۻ������в������Ӽ� | |
| B�� | ���ǽ���Ԫ�ظ��ǽ���Ԫ�ػ����γ����ӻ����� | |
| C�� | ���ӻ������е������Ӷ��ǽ������� | |
| D�� | ����ˮ���Ե���Ļ�����һ�������ӻ����� |