题目内容
(1)现有以下物质:①NaCl晶体 ②SO2 ③稀硫酸 ④石墨 ⑤BaSO4固体 ⑥蔗糖(C12H22O11) ⑦酒精 ⑧熔融的KNO3 ⑨CaO ⑩纯净的醋酸
请回答下列问题(用序号):
以上物质中能导电的是 ;以上物质中属于电解质的是 。
(2)按要求写出下列对应的方程式:
(①电离方程式、②化学方程式、③离子方程式)
①Al2(SO4)3:
②CO2+2OH-=CO32-+H2O:
③NaHCO3与NaHSO4溶液反应:
(1)③④⑧ ①⑤⑧⑨⑩
(2)①Al2(SO4)3=2Al3++3SO42- ②CO2+2NaOH= H2O +Na2CO3 ③HCO3-+ H+=CO2↑+H2O
解析试题分析:(1)②⑥⑦为非电解质,③④既不是电解质又不是非电解质。(2)Al2(SO4)3为盐,是强电解质,完全电离。②可以是NaOH或者KOH。③NaHCO3电离出HCO3- , NaHSO4电离出H+。
考点:电解质的基本概念和离子方程式的书写。

 智趣暑假温故知新系列答案
智趣暑假温故知新系列答案 英语小英雄天天默写系列答案
英语小英雄天天默写系列答案 暑假作业安徽少年儿童出版社系列答案
暑假作业安徽少年儿童出版社系列答案溴及其化合物广泛应用在有机合成、化学分析等领域。
(1)海水提溴过程中溴元素的变化如下: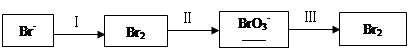
①过程Ⅰ,海水显碱性,调其pH<3.5后,再通入氯气。
ⅰ.通入氯气后,反应的离子方程式是______。
ⅱ.调海水pH可提高Cl2的利用率,用平衡原理解释其原因是______。
②过程Ⅱ,用热空气将溴赶出,再用浓碳酸钠溶液吸收。完成并配平下列方程式。
Br2+ Na2CO3=
Na2CO3= NaBrO3+
NaBrO3+ CO2+
CO2+ ______
______
③过程Ⅲ,用硫酸酸化可得Br2和Na2SO4的混合溶液。
相同条件下,若用盐酸酸化,则所得溴的质量减少,原因是______。
(2)NaBrO3是一种分析试剂。向硫酸酸化的NaI溶液中逐滴加入NaBrO3溶液,当加入2.6 mol NaBrO3时,测得反应后溶液中溴和碘的存在形式及物质的量分别为:
| 粒子 | I2 | Br2 | IO3- |
| 物质的量/mol | 0.5 | 1.3 | |
二氧化硫和氮氧化物(NOx)对大气污染日趋严重,研究消除大气污染的方法是化学工作者的重要课题,目前有很多种方法消除大气污染。
(1)可利用甲烷催化还原NOx的方法处理NOx,反应如下:
CH4(g)+4NO2(g)= 4NO(g)+CO2(g)+2H2O(g);△H= -574 kJ·mol-1
CH4(g)+4NO(g)= 2N2(g)+CO2(g)+2H2O(g);△H= -1160 kJ·mol-1
则CH4(g)+2NO2(g)= N2(g)+CO2(g)+2H2O(g);△H= ;
(2)降低汽车尾气的方法之一是在排气管上安装催化转化器,发生如下反应:2NO(g)+2CO(g) N2(g)+2CO2(g);△H<0。
N2(g)+2CO2(g);△H<0。
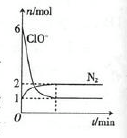
若在一定温度下,将2molNO、1molCO充入1L固定容积的容器中,15分钟后达到平衡,反应过程中各物质的浓度变化如图甲所示,则平衡常数K= (小数点后保留3位);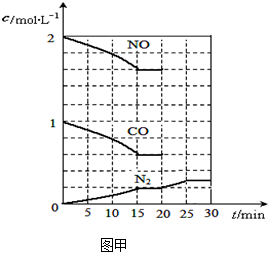
①若保持温度不变,20min时再向容器中充入CO气体0.6mol,平衡将 移动(填“向左”、“向右”或“不”);
②若保持温度不变,20min时向原平衡容器中充入CO、N2各0.6mol,平衡将 移动(填“向左”、“向右”或“不”);
③20min时,若改变反应条件,导致N2浓度发生如图所示的变化,则改变的条件可能是
(填字母);
| A.加入催化剂 | B.降低温度 | C.缩小容器体积 | D.增加CO2的量 |
有M、N两种溶液,经测定这两种溶液中含有下列12种离子:Al3+、Cl-、Na+、K+、NO3-、OH-、Fe2+、AlO2-、CO32-、NH4+、SO42-、H+。
(1)完成下列表格中实验①的结论和实验②的实验内容以及现象:
| 实验内容以及现象 | 结论 |
| ①取少量N溶液滴加足量的硝酸钡溶液,无沉淀产生 | N中不含 离子 |
| ② | 确定M溶液中含有Na+,不含K+ |
 ③用pH试纸检测M溶液,pH试纸呈蓝色 ③用pH试纸检测M溶液,pH试纸呈蓝色 | |
NO3-存在于________溶液中,理由是____________________________________;
Cl-存在于________溶液中,是根据_________________ 确定的。
(3)根据(1)中的实验确定,M溶液中含有的离子为________________________。