��Ŀ����
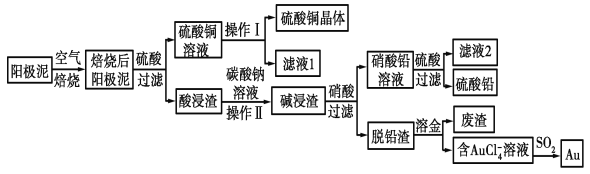
����Ŀ����ͭ��������������к���Cu��Au(��)��PbSO4�����ʣ�ʪ����������������ۺ����õĹ���������ͼ��ʾ��
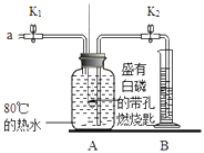
��1����⾫����ͭ����Ǧ�Ĵ�ͭʱ�����ҺӦ����________��Һ�����Һ�����ʱ�����ĵ缫��ӦʽΪ___________________________��Cu��2e��===Cu2����
��2����ɲ���������Ҫ�����У�__________________�����ˣ�ϴ�ӣ����
��3��д����SO2��ԭAuCl4-�����ӷ�Ӧ����ʽ____________________________��
��4��Ϊ�˼��ٷ�Һ�ŷš��������������Դ����ҵ�Ͻ���Һ1��������ͭ��Һ����ѭ����������ָ������ͼ����һ�����Ƶ�����________________________��
��5�������ӷ���ʽ��ʾ����̼������Һ�����ã�___________________________��[��֪298 Kʱ��Ksp(PbCO3)��1.46��10��13��Ksp(PbSO4)��1.82��10��8]������Һ��c(SO42-)=0.2mol/Lʱ��c(CO32-)=_______________mol/L�����������2λ��Ч���֣�
���𰸡�CuSO4 Pb-2e-+SO42-=PbSO4 ����Ũ������ȴ�ᾧ 3SO2+2AuCl4-+6H2O=2Au+3 SO42-+8Cl-+12H+ ����Һ2�ܽ������������������ܽ������� PbSO4��s��+CO32-(aq)![]() PbCO3��s��+SO42-(aq) 1.6��10-6
PbCO3��s��+SO42-(aq) 1.6��10-6
��������
��ͭ��������������к���Cu��Au(��)��PbSO4�����ʣ����պ����ͭ��Ϊ����ͭ��������Եõ�����ͭ��Һ������ͭ��Һ��������Ũ�������½ᾧ�����ˡ�ϴ��������������ͭ������Au(��)��PbSO4�����ʾ�̼���ƽ�ϴ��Ũ���������������˵õ�����Ǧ��Һ����Һ��������������Ǧ�������ٹ��˵õ�����Ǧ����Ǧ������Ҫ�ǽ�������ˮ�ܽ����õ�����AuCl4-����Һ��AuCl4-���Ա�SO2��ԭ�õ�Au���Դ˽��
��ͭ��������������к���Cu��Au(��)��PbSO4�����ʣ����պ����ͭ��Ϊ����ͭ��������Եõ�����ͭ��Һ������ͭ��Һ��������Ũ�������½ᾧ�����ˡ�ϴ��������������ͭ������Au(��)��PbSO4�����ʾ�̼���ƽ�ϴ��Ũ���������������˵õ�����Ǧ��Һ����Һ��������������Ǧ�������ٹ��˵õ�����Ǧ����Ǧ������Ҫ�ǽ�������ˮ�ܽ����õ�����AuCl4-����Һ��AuCl4-���Ա�SO2��ԭ�õ�Au��
(1)��⾫����ͭʱһ��������ͭ��Һ���������Һ����⾫���Ĵ�ͭ�������ᷢ��������Ӧ�����е���ͭ�ͻ����Ա�Cuǿ�Ľ������ᷢ���ܽ�����˴�ͭ�е�ͭ��Ǧ�ᷢ��ʧ���ӵ�������Ӧ���缫��ӦʽΪ��Pb-2e-+SO42-=PbSO4��
��ˣ�������ȷ������CuSO4��Pb-2e-+SO42-=PbSO4��
(2) ����I�IJ����Ǵ�����ͭ��Һ�л������ͭ��������˸ò���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ�����
��ˣ�������ȷ����������Ũ�������½ᾧ��
(3) SO2��ԭAuCl4-�л�ԭ�������������Ƚ���ȷ����˺������Ʋ������������SO42-����ԭ������Au������������ԭ��Ӧ��ʧ�����غ���ȱ����ƽ��Ȼ����ݵ���غ�����ƽ����˵õ��ķ�Ӧ����ʽΪ��3SO2+2AuCl4-+6H2O=2Au+3 SO42-+8Cl-+12H+��
��ˣ�������ȷ������3SO2+2AuCl4-+6H2O=2Au+3 SO42-+8Cl-+12H+��
(4) ��Һ1���ڽᾧ����ͭʱʣ�µ���Һ��������������δ����������ͭ����˲���ǰ�������ͭ��Һ����ѭ���������ڳ��������������ƴ˴���������Ӧ���������еõ�����һ��Һ2������Һ2��������Ǧ��Һ�м���������������Ǧ����������������Ǧ��ʣ�µ���Һ������Һ��H+û�з�����Ӧ����˻��д�����������Һ�����Կ��Ѵ���Һ�����������ǰ��ļ�������ܽ�������
��ˣ�������ȷ���ǣ�����Һ2�ܽ������������������ܽ���������
(5) ͨ���Ƚ����ֳ������ܶȻ������Կ���̼��Ǧ������Ǧ�������������������Ǧ�м���̼�������������dz����ܽ�ת���ķ�Ӧ�����ӷ���ʽҪע�����״̬������ʽΪ��PbSO4��s��+CO32-(aq)![]() PbCO3��s��+SO42-(aq)��
PbCO3��s��+SO42-(aq)��
����Һ��c(SO42-)=0.2mol/Lʱ��c(CO32-)=![]() ��c(SO42-)=
��c(SO42-)=![]() ��0.2mol/L=1.6��10-6mol/L��
��0.2mol/L=1.6��10-6mol/L��
��ˣ�������ȷ������PbSO4��s��+CO32-(aq)![]() PbCO3��s��+SO42-(aq)��1.6��10-6��
PbCO3��s��+SO42-(aq)��1.6��10-6��

 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�