��Ŀ����
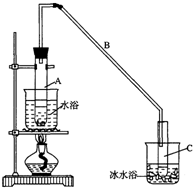
13��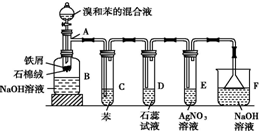 ��1���������ȡ����Ӧ��ʵ��װ����ͼ��ʾ������AΪ��֧�Թܸ��Ƴɵķ�Ӧ�����������¶˿���һС�ף�����ʯ���ޣ��ټ���������м��
��1���������ȡ����Ӧ��ʵ��װ����ͼ��ʾ������AΪ��֧�Թܸ��Ƴɵķ�Ӧ�����������¶˿���һС�ף�����ʯ���ޣ��ټ���������м����д���пհף�
���Թ�C�б��������ǣ�����Br2������
��Ӧ��ʼ�۲�D��E���Թܣ�����������Ϊ��D���б�죬E���г���dz��ɫ������
�ڷ�Ӧ2��3min����B�е�NaOH��Һ��ɹ۲쵽�������ǣ�
�ײ������״Һ�壮
������������װ���У����з�������������F������ĸ����
��2��ʵ�����Ʊ�����������Ҫ�������£�
a������һ��������ŨH2SO4��ŨHNO3�Ļ���ᣬ���뷴Ӧ���У�
b���������µĻ��������μ���һ�����ı����������Ͼ��ȣ�
c����55�桫60���·�����Ӧ��ֱ����Ӧ������
d����ȥ�����ֲ�Ʒ����������ˮ��5% NaOH��Һϴ�ӣ������������ˮϴ�ӣ�
e��������ˮCaCl2�����Ĵ��������������õ�����������������д���пհף�
������һ��������ŨH2SO4��ŨHNO3�Ļ����ʱ��������ע�������ǣ��Ƚ�Ũ����ע�������У�������ע��Ũ���ᣬ����ʱ�������ȴ��
�ڲ���d��ϴ�ӡ������������Ӧʹ�õ������Ƿ�Һ©����
�۲���d�дֲ�Ʒ��5% NaOH��Һϴ�ӵ�Ŀ���ǣ���ȥ�ֲ�Ʒ�в������ᣮ
���Ʊ��������Ļ�ѧ����ʽ��
 ��
��
���� ��1�����屽�е����ӷ����Ǽ��Է��ӵ����������ڷǼ��Է��ӵ��ܼ����ݴ˷����������ã��÷�Ӧ�����廯�����ɣ��廯������ˮ�õ������ᣬ��������ʹʯ����Һ���ɫ���������ܺ���������Ӧ���ɵ���ɫ�����廯����
���屽���л�������������������ڣ����ܶȴ�������������Һ��
�۵���©�������θ�����ܷ�ֹ��Һ������
��2����Ũ������Ũ�����Ϸų��������ȣ����ƻ���Ӧ��Ũ������ע��Ũ�����У���ʱ���衢��ȴ����ֹ�������ˣ�
�ڷ��뻥�����ܵ�Һ̬����ȡ��Һ��������Ҫ�÷�Һ©����
�۷�Ӧ�õ��ֲ�Ʒ���в��������ἰ���ᣬ��Ҫ��ȥ��
�ܱ��������ᷢ��ȡ����Ӧ�õ���������
��� �⣺��1�����屽�е����ӷ���������Ȼ�̼���ǷǼ��Է��ӣ�������������ԭ��֪�������������Ȼ�̼���������Ȼ�̼���������������������÷�Ӧ�����廯�����ɣ��廯������ˮ�õ������ᣬ���������������ʣ���ʹʯ����Һ���ɫ���������ܺ���������Ӧ���ɵ���ɫ�����廯�������Թ۲�D��E���Թܣ�������������D���б�죬E���г���dz��ɫ������
�ʴ�Ϊ������Br2������D���б�죬E���г���dz��ɫ������
���屽���л������������Һ����������屽������������Һ�����ܣ����屽���ܶȴ���ˮ���ܶȣ�������B�е�����������Һ��ɹ۲쵽�������ǵײ������״Һ�壬
�ʴ�Ϊ���ײ������״Һ�壻
�۵�����ѹ������ѹ���仯ʱ��Һ����������������Ϊ���õ�©���¿ںܴ�Һ��������С�ĸ߶Ⱦ��кܴ�������������Һ��ı�����ѹ�����ɵͳ�ѹ���IJ����⣮��������ϲ����п������룬Һ�岻�ᵹ�����϶˵�ϸ�ܵ������Ծ��з��������õ�������F��
�ʴ�Ϊ��F��
��2����Ũ������Ũ�����Ϸų��������ȣ����ƻ������ע�������ǣ��Ƚ�Ũ����ע�������У�������ע��Ũ���ᣬ����ʱ�������ȴ��
�ʴ�Ϊ���Ƚ�Ũ����ע�������У�������ע��Ũ���ᣬ����ʱ�������ȴ��
������������״Һ�壬��ˮ�����ܣ����뻥�����ܵ�Һ̬����ȡ��Һ��������Ҫ�÷�Һ©����
�ʴ�Ϊ����Һ©����
�۷�Ӧ�õ��ֲ�Ʒ���в��������ἰ���ᣬ������������Һϴ�ӳ�ȥ�ֲ�Ʒ�в������ᣬ
�ʴ�Ϊ����ȥ�ֲ�Ʒ�в������
�ܱ��������ᷢ��ȡ����Ӧ�õ��������������ɱ���ȡ�������Ļ�ѧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�����л����Ʊ�ʵ�飬�漰����������ȡ�������ڶԻ���ʵ������Ŀ��飬��Ŀ�ѶȲ���

| A�� | M+ | B�� | HA- | C�� | A2- | D�� | M+��HA- |
 ijʵ��С����������װ�úϳ�����ȩ�������ķ�Ӧ���£�
ijʵ��С����������װ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/�� | �ܶ�/g•cm-3 | ˮ���ܽ��� | |
| ������ | 117.2 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У���A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����ϵ���֣�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g���ش��������⣺
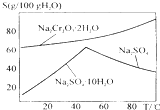
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵�����ɲ��ܣ�Ũ��������ˮ��ų������ȣ����������ˣ�
��2�������ʯ�������Ƿ�ֹ���У�
��3������װ��ͼ�У�B�����������Ƿ�Һ©����D������������ֱ�������ܣ�
��4����Ӧ�¶�Ӧ������90��95�棬��ԭ���DZ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
��5���¶ȼ�C1�����ò�����ƿ�з�Ӧ����¶ȣ�C2�����ò�����ֵķе㣮
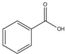 ��Ϊԭ�ϣ���ȡ�������������֪�й����ʵķе����±���
��Ϊԭ�ϣ���ȡ�������������֪�й����ʵķе����±���| ���� | �״� | ������ | ��������� |
| �е�/�� | 64.7 | 249 | 199.6 |
��Բ����ƿ�м���12.2g�����ᣨM=122g/mol����20ml�״����ܶ�Լ0.79g•mL-1 ������С�ļ���3mLŨ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ��
��1������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽC6H5CO18OH+CH3OH$?_{��}^{ŨH_{2}SO_{4}}$C6H5COOCH3+H218O��Ũ����������ǣ���������ˮ����
��2���ס��ҡ�����λͬѧ�ֱ����������ͼ����ʵ���Һϳɱ����������װ�ã��г������ͼ�������������ȥ���������л���ķе㣬��ò�����װ�ã���ס����ҡ���������
�ֲ�Ʒ�ľ���
��3������������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ��������������̽��о��ƣ����������ͼд���������������ƣ��������Һ ����������
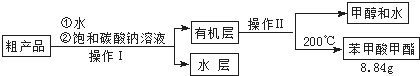
��4���ܷ���NaOH��Һ���汥��̼������Һ������ܡ�����������ԭ������������ǿ��ٽ������������ˮ�⣬���²�Ʒ��ʧ��
��5��ͨ�����㣬����������IJ�����65%��
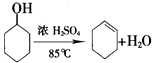
 ����ϩ��һ����Ҫ�Ļ���ԭ�ϣ�
����ϩ��һ����Ҫ�Ļ���ԭ�ϣ���1��ʵ���ҿ��ɻ������Ʊ�����ϩ����Ӧ�Ļ�ѧ����ʽ��
 ��
����2��ʵ��װ����ͼ��ʾ����10mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
�������ͻ���ϩ�IJ��������������£�
| �ܶ� ��g/cm3�� | �۵� ���棩 | �е� ���棩 | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
���Թ�A����ˮԡ�е�Ŀ�������Ⱦ��ȣ����ڿ��£��Թ�C���ڱ�ˮԡ�е�Ŀ����ʹ����ϩҺ�������ٻӷ���
��3������ϩ��Ʒ�к����������������������ʣ����ƻ���ϩ�ķ����ǣ�
����ϩ��Ʒ�м���C�������ţ��������Һ����������ƣ���
A��Br2��CCl4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٶԳ������Ӻ�Ļ���ϩ�������õ�����ϩ��Ʒ������ʱ��������ƿ��Ҫ����������ʯ�ң�Ŀ���dz�ȥ��Ʒ��������ˮ��
��ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����C��
A������ʱ��70�濪ʼ�ռ���Ʒ
B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
��4���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������B��
A������ˮ�۲�ʵ������
B����������ƹ۲�ʵ������
C���������Ը��������Һ����۲�ʵ������

�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | һl30 | 9 | -1l6 |
��1���йط�Ӧ����ʽ��CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O��CH2=CH2+Br2��BrCH2CH2Br��
��2����װ��C��Ӧ����c������ȷѡ��ǰ����ĸ������Ŀ�������շ�Ӧ�п������ɵ���������
a��ˮ ���� b��Ũ���� �� c������������Һ d������̼��������Һ
��3���жϸ��Ʊ�����Ӧ�Ѿ�������������������ɫ��ȫ��ȥ��
��4�������������������������ѣ���������ķ�����ȥ��
��5����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ȴ�ɱ�����Ĵ����ӷ������ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���Dz�Ʒ1��2-����������۵㣨���̵㣩�ͣ�������ȴ�����̶��������ܣ�
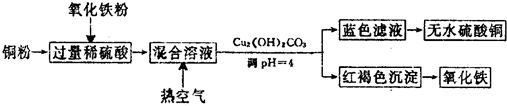
 ��ҵ���Ը�������Ҫ�ɷ�FeO•Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7•2H2O������Ҫ��Ӧ���£�
��ҵ���Ը�������Ҫ�ɷ�FeO•Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7•2H2O������Ҫ��Ӧ���£�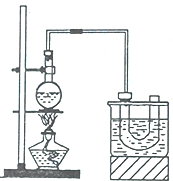 ��ʵ���������ɵ�����£�ʵ����Ҳ���Խ���ijЩ�л�����Ʊ���ʵ�����Ʊ������飨C2H5Br����װ�úͲ������£���֪������ķе�38.4�棬�ܶȱ�ˮ��������ˮ����
��ʵ���������ɵ�����£�ʵ����Ҳ���Խ���ijЩ�л�����Ʊ���ʵ�����Ʊ������飨C2H5Br����װ�úͲ������£���֪������ķе�38.4�棬�ܶȱ�ˮ��������ˮ����