��Ŀ����
19��������Ҫ��������ԭ�ϣ�����һ������ҹ�Ŀǰ�Ƶ����Ҫ������ij�о���ѧϰС��������ף���Ʋ�����������ģ��ʵ�飮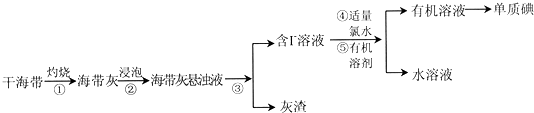
��ش�
��1������������պ����õ���ʵ��������b������ţ���
a���Թ� b������ c���ձ�
��2������۵�ʵ������ǹ��ˣ�
��3��������з�Ӧ�����ӷ���ʽ��Cl2+2I-=2Cl-+I2��
��4��������У�����ѡ����л��ܼ���bd������ţ���
a������ b�����Ȼ�̼ c���ƾ� d����
��5��ͬѧ�ǹ۲쵽��ɲ���ݺ����õ���ˮ��Һ�ʵ���ɫ��ijͬѧ�ƶ����п��ܺ��еⵥ�ʣ����鷽���ǣ�ȡ������Һ���Թ��У����뼸�ε�����Һ������Һ����ɫ��֤����Һ�л�����I2��
��6���������Cl2����I2��Ӧ�����Բ�����м������ˮӦ����������Ϊ֤����˵����ijͬѧ������ʵ�飺ȡ������ˮ���μ���ˮ���۲쵽��ˮ����ɫ�������������ﺬ��IO3-���÷�Ӧ�Ļ�ѧ����ʽ��5Cl2+I2+6H2O�T2HIO3+10HCl��
���� �ɺ������յõ������ҽ��ݵõ�����������Һ�����˵õ���������Һ����Һ�к���I-������������ˮ����������Ϊ�ⵥ�ʣ������л��ܼ�����ȡ��Һ�õ��л��㣬����õ��ⵥ�ʣ�
��1������ʵ���������������������õ���ʵ��������
��2����������Һ���ù��ˣ���
��3������������Һͨ������Cl2��Ϊ�˽������������ɵ��ʵ⣬���ӷ���ʽΪ2I?+Cl2=I2+2Cl-��
��4��������ȡ��ѡ���ԭ������жϣ���ע�⣺ѡ�������ȡ����Լ����˶�I2��ǿ���ܽ������⣬������������ˮ�������ܽ��з�Һ���룻
��5���������۱���ɫ����6�����ݹ�����Cl2����I2��Ӧ������IO3-�жϻ�ԭ�������ƽ��
��� �⣺��1�����չ�������һ��ʹ��������������������Ҫ��������֧��Ȼ��������ż��ϣ����ż�����žƾ��ƣ��ʴ�Ϊ��b��
��2��������Һ����Ϊ�����ͺ���������ҺӦѡ����˵ķ������ʴ�Ϊ�����ˣ�
��3������������Һ�м�����������ͨ������Cl2��Ϊ�˽������������ɵ��ʵ⣬���ӷ���ʽΪ2I?+Cl2=I2+2Cl-���ʴ�Ϊ��2I-+Cl2=I2+2Cl-��
��4�����õ��������л��ܼ���������������ȡ����ע�⣺ѡ�������ȡ����Լ����˶�I2��ǿ���ܽ���������������������ˮ�������ܽ��з�Һ���룬���������������Ȼ�̼�ͱ����ʴ�Ϊ��bd��
��5�����ݵ����������ɫ����ⵥ�ʵĴ��ڣ�ʵ�����Ϊȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣬
�ʴ�Ϊ��ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣩��
��6���������Cl2����I2��Ӧ������IO3-���ʸ÷�Ӧ�Ļ�ѧ����ʽ��5Cl2+I2+6H2O�T2HIO3+10HCl���ʴ�Ϊ��5Cl2+I2+6H2O�T2HIO3+10HCl��
���� ���⿼���Ʊ�±�ص����ʡ����ʷ������ᴿ�IJ�������Ŀ�Ѷ��еȣ�ע�����ʵ�������Ҫ���ע�����

 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�| A�� | ���³�ѹ�£�32g����������ԭ����ΪNA | |
| B�� | ��״���£�22.4L ���Ȼ�̼�ķ�����ΪNA | |
| C�� | 0�棬101KPa�£�44.8L���������еķ�����Ϊ2NA | |
| D�� | ���³�ѹ�£�1mol�������еķ�����Ϊ2NA |
| A�� | ����Ϊ�������־��Բ����ܣ���ΪH3����Υ���˹��ۼ����� | |
| B�� | ����Ϊ��������ķ��ִ��ڣ���֤����ͳ�ļۼ����۲������� | |
| C�� | ����ΪH3����ʵ������H2������H+������ۼ���ϵIJ��Ӧд��H3+ | |
| D�� | ����Ϊ�����л����ܴ�����һ���ⵥ�ʣ���Ϊ��Ԫ��������ͬλ�ر�Ȼ������ͬ�������� |
| A�� | 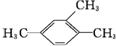 1��3��4-���ױ� 1��3��4-���ױ� | B�� | 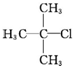 ��2-��-2-�ȱ��� ��2-��-2-�ȱ��� | C�� | 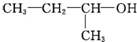 ��2-���� ��2-���� | D�� | 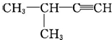 ��2-��-3-��Ȳ ��2-��-3-��Ȳ |
| A�� | ��100-$\frac{7A}{6}$��% | B�� | 10A% | C�� | ��$\frac{A}{6}$��% | D�� | 6A% |
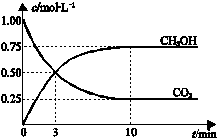 ��֪��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1��һ�������£������Ϊ1L���ܱ������г���1mol CO2��3mol H2�����CO2��CH3OH��g����Ũ����ʱ��仯������ͼ��ʾ�����������У���ȷ���ǣ�������
��֪��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1��һ�������£������Ϊ1L���ܱ������г���1mol CO2��3mol H2�����CO2��CH3OH��g����Ũ����ʱ��仯������ͼ��ʾ�����������У���ȷ���ǣ�������| A�� | �����¶���ʹ$\frac{n��C{H}_{3}OH��}{n��C{O}_{2}��}$���� | |
| B�� | ��Ӧ�ﵽƽ��״̬ʱ��CO2��ƽ��ת����Ϊ75% | |
| C�� | 3 minʱ����CO2��Ũ�ȱ�ʾ������Ӧ���ʵ�����CH3OH��Ũ�ȱ�ʾ���淴Ӧ���� | |
| D�� | �ӷ�Ӧ��ʼ��ƽ�⣬H2��ƽ����Ӧ���ʦԣ�H2��=0.075mol/��L��min�� |
| A�� | 1mol��̬���ʻ�Һ̬���ʵ������Ҫ�����ڹ����������ʵ����ӵĴ�С | |
| B�� | ����ͬ�¶Ⱥ�ѹǿ�£��κ���������֮��ľ���ɿ�����ȵ� | |
| C�� | ijͬѧ������ƿ������Һ��ˮʱ���������̶��߱����������� | |
| D�� | Ϊ������ƿ�ľ�����ƥ�������������ʱӦ��������ƽ |
 ���200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⣮
���200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⣮