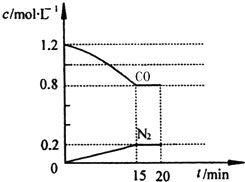
��Ŀ����
4������������Ϊ98%���ܶ�Ϊ1.84g•cm-3��Ũ�������Ƴ�200mL 1.84mol/L��ϡ���ᣬ�ṩ�������У�A.100mL��Ͳ��B.20mL��Ͳ��C.200mL����ƿ��D.500mL����ƿ��E.50mL�ձ���F����ͷ�ιܣ�G������������1�����Ƹ���Һ��Ӧȡ��Ũ��������Ϊ20mL��
��2��ʵ����������У�Ӧѡ���������Ⱥ�˳����BEGCF������ţ���
��3�����ƹ��������������������������ϡ�����Ũ����ʲôӰ�죨�ƫ����ƫС������Ӱ�족����
������Ͳ��ȡŨ����ʱ��������Ͳ�̶���ƫС��
�����������IJ�����û��ϴ��ƫС��
�۶���ʱ�����ӹ۲�����ƿ�̶���ƫС��
�ܶ��ݺ�ҡ�ȣ�����Һ����ڿ̶��ߣ��ֵμ�ˮ��Һ����̶��߸պ���ƽƫС��
���� ��1���������ʵ���Ũ������������������ת����ϵc=$\frac{1000�Ѧ�}{M}$�������Ũ�����Ũ�ȣ�Ȼ��������ƹ��������ʵ����ʵ�������������ҪŨ����������
��2����������һ�����ʵ�����Ũ�ȵ���Һ�����ʹ�õ�������������
��3������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1����������Ϊ98%���ܶ�Ϊ1.84g•cm-3��Ũ�������ʵ���Ũ��Ϊ��$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L������200mL 1.84mol/L��ϡ���ᣬ���ƹ�������������ʵ������䣬�����ĸ�Ũ��������Ϊ��$\frac{1.84mol/L��0.2L}{18.4mol/L}$=0.02L=20mL��
�ʴ�Ϊ��20mL��
��2������200mL 1.84mol/L��ϡ����IJ����У���20ml��Ͳ��ȡ20mLŨ���ᣬ����ȡ��Ũ�������ձ���ϡ�ͣ�ͬʱ�õ����������裬��ȴ��ϡ�͵�Ũ����ת�Ƶ�200mL����ƿ�У�Ȼ��ϴ���ձ��Ͳ���������ֱ�Ӽ�������ˮ����������Һ��1-2cmʱ���ý�ͷ�ιܶ��ݣ����ҡ�ȣ�����ʹ���������Ⱥ�˳��Ϊ��BEGCF��
�ʴ�Ϊ��BEGCF��
��3��������Ͳ��ȡŨ����ʱ��������Ͳ�̶��ߣ���ȡ��Ũ�������ƫС�����Ƶ���ҺŨ��ƫС��
�ʴ�Ϊ��ƫС��
�����������IJ�����û��ϴ�ӣ��������Ƶ���Һ����������ʵ���ƫС�����Ƶ���ҺŨ��ƫС��
�ʴ�Ϊ��ƫС��
�۶���ʱ�����ӹ۲�����ƿ�̶��ߣ����¼��������ˮ���ƫ�����Ƶ���Һ���ƫ����ҺŨ��ƫС��
�ʴ�Ϊ��ƫС��
�ܶ��ݺ�ҡ�ȣ�����Һ����ڿ̶��ߣ��ֵμ�ˮ��Һ����̶��߸պ���ƽ���������Ƶ���Һ���ƫ����ҺŨ��ƫС��
�ʴ�Ϊ��ƫС��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ�������Ŀ�ѶȲ�����ȷ����һ�����ʵ���Ũ�ȵ���Һ����Ϊ���ؼ���ע�������������ķ����뼼�ɣ�

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�| A�� | YԪ�ؿ���+6�� | |
| B�� | X��Y��Z����Ԫ����ɵĻ����ﻯѧʽ������X2YZ3��X2YZ4�� | |
| C�� | X��Y��Z����Ԫ����ɵĻ����ﲻһ�������� | |
| D�� | Y�ĵ縺�Ա�Z�� |
| A�� | ��ʯī���缫��ⱥ��ʳ��ˮʱ���������õ�2NA�����ӣ�����������22.4L���� | |
| B�� | ���³�ѹ�£�Na2O2������H2O��Ӧ��������0.2mol O2��ת�Ƶĵ�����Ŀ��0.8NA | |
| C�� | 1L 1mol/L��K2CO3��Һ����������С��3NA | |
| D�� | �����£�42.0g ��ϩ�ͱ�ϩ�Ļ�������к��е�̼ԭ����Ϊ3NA |
| A�� | ��ⱥ��ʳ��ˮʱ�������ĵ缫��ӦʽΪ��2Cl--2e-�TCl2�� | |
| B�� | �ڶƼ��ϵ��ͭʱ���öƼ����������缫��ӦʽΪ��Cu2++2e-�TCu | |
| C�� | ��ͭ����ʱ�����Դ�����������Ǵ�ͭ���缫��ӦʽΪ��Cu-2e-�TCu2+ | |
| D�� | ��������������ʴʱ��������ӦʽΪ��O2+2H2O+4e-�T4OH- |
��
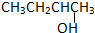 ��CH3CH2CH2CH2OH��
��CH3CH2CH2CH2OH��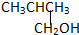 ��
��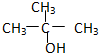
������������ȩ���ǣ�������
| A�� | �٢� | B�� | ֻ�Т� | C�� | �ں͢� | D�� | �ۺ͢� |
| A�� | �ڶ�����Ԫ�ص�������۴����������δ�+1������+7�� | |
| B�� | ͬһ���ڴ����ң�Ԫ�صĽ���������ǿ���ǽ��������� | |
| C�� | ��IAԪ��ȫ���ǽ���Ԫ�� | |
| D�� | ���ڽ�����ǽ����ֽ��߸���Ѱ�Ұ뵼����� |