��Ŀ����
����Ŀ����1���ϳɰ���Ӧ��ʹ�õĴ�����______�������ƣ����÷�Ӧ�¶�һ�������500�棬��Ҫԭ����______��
��2�����д�ʩ�����ܼӿ�ϳɰ���Ӧ�ķ�Ӧ���ʣ���������Ӧ��ת���ʵ�����_____��
A. ʹ�ô��� B. ��С�ݻ���� C. ��߷�Ӧ�¶� D. ����NH3
��3����t2ʱ�̣����������ݻ�Ѹ������ԭ����2���������������²��������£�t3ʱ�̴ﵽ�µ�ƽ��״̬��֮���ٸı�������������ͼ�в��仭����t2��t4ʱ������Ӧ������ʱ��ı仯����__________��
��4�������£��ڰ�ˮ�м���һ�������Ȼ�茶��壬����˵�����������________��
A.��Һ��pH���� B.��ˮ�ĵ���̶ȼ�С C.c(NH4+)��С D.c(OH��)��С
��5��ʯ���HZ��ʾ����Һ�д��ڵĵ���ƽ��HZ����ɫ��HZ����ɫ����ͨ�백����ʯ����Һ����ɫ������ƽ���ƶ�ԭ������____________��
���𰸡�����ý ������������ҷ�Ӧ�¶ȽϿ� B 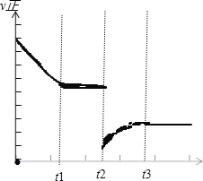 AC ͨ�백��������һˮ�ϰ���һˮ�ϰ�����������������������ӽ�ϣ�ʹ������Ũ�ȼ�С����ʹƽ�������ƶ�������ɫ�������ӣ������Һ����ɫ
AC ͨ�백��������һˮ�ϰ���һˮ�ϰ�����������������������ӽ�ϣ�ʹ������Ũ�ȼ�С����ʹƽ�������ƶ�������ɫ�������ӣ������Һ����ɫ
��������
(1)�ϳɰ���ҵ������ý�������������¶ȿ�����Ӧ���ʣ���߲��������¶�ʱ�������Ļ����������������Ӧ���ʣ���߲������ʴ�Ϊ������ý�������Ļ����¶��ҷ�Ӧ���ʽϿ죻
(2)A.ʹ�ô������ӿ��˷�Ӧ���ʣ����Dz�Ӱ�컯ѧƽ�⣬��Ӧ���ת���ʲ��䣬��A����
B. ��С���������������ѹǿ�������˷�Ӧ���ʣ�ƽ�����������ƶ�����Ӧ��ת��������B��ȷ��
C. ��߷�Ӧ�¶ȣ������˷�Ӧ���ʣ�����ƽ�����������ƶ�����Ӧ��ת���ʼ�С����C����
D. ���߰�������Ӧ���С�����淴Ӧ���ʶ�����С����D����
�ʴ�Ϊ��B��
��3�����������ݻ�Ѹ������ԭ����2�����������������������£��൱�ڼ�Сѹǿ�����淴Ӧ���ʶ���ԭ����С��ƽ�������ƶ�����V����V������ͼ��Ϊ��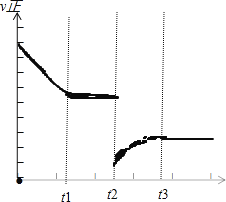 ��
��
(4)A.��ˮ�м����Ȼ�泥���Һ�������Ũ������������һˮ�ϰ��ĵ��룬��Һ������������Ũ�ȼ�С����Һ��pH��С����A����
B. ��ˮ�������Ũ������һˮ�ϰ��ĵ���ƽ�����������ƶ�������ˮ�ĵ���ȼ�С����B��ȷ��
C. �������Ȼ�泥���Һ�������Ũ������C����
D. ��ˮ�е������Ũ��������Һ������������Ũ�ȼ�С��c(OH��)��С����D��ȷ��
�ʴ�Ϊ��AC��
��5��ͨ�백������Һ�Լ��ԣ����������ӣ�ʹƽ�����ƣ���Һ����ɫ��



