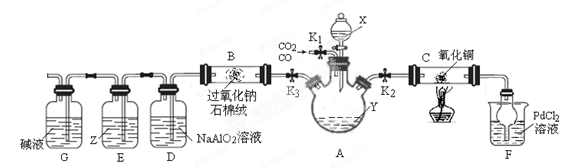
ЬтФПФкШн
ШчЭМЫљЪОЁАКЯГЩАБЁБЕФбнЪОЪЕбщ(МаГжвЧЦїОљвбЪЁТд)ЁЃдкYаЮЙмЕФвЛВргУZnСЃКЭЯЁH2SO4ЗДгІжЦШЁH2ЃЌСэвЛВргУNaNO2ЙЬЬхКЭNH4ClБЅКЭШмвКЗДгІжЦШЁN2ЃЌN2КЭH2ЛьКЯКѓЭЈЙ§ЛЙдЬњЗлРДКЯГЩNH3ЃЌдйНЋВњЩњЕФЦјЬхЭЈШыЗгЬЊЪдвКжаЃЌШєЗгЬЊЪдвКБфКьЃЌдђЫЕУїВњЩњСЫАБЦјЁЃ

ФГПЮЭтЛюЖЏаЁзщЭЈЙ§ВщдФзЪСЯКЭЖрДЮЪЕбщЃЌЕУЕНСЫШчЯТаХЯЂЃК
аХЯЂвЛЃКNaNO2ЙЬЬхКЭБЅКЭNH4ClШмвКЛьКЯМгШШЕФЙ§ГЬжаЗЂЩњШчЯТЗДгІЃК
ЂйNaNO2ЃЋNH4Cl NH4NO2ЃЋNaCl
NH4NO2ЃЋNaCl
ЂкNH4NO2 NH3ЁќЃЋHNO2
NH3ЁќЃЋHNO2
Ђл2HNO2 N2O3ЁќЃЋH2O
N2O3ЁќЃЋH2O
Ђм2NH3ЃЋN2O3 2N2ЃЋ3H2O
2N2ЃЋ3H2O
аХЯЂЖўЃКВщдФзЪСЯЃЌВЛЭЌЬхЛ§БШЕФN2ЁЂH2ЛьКЯЦјЬхдкЯрЭЌЪЕбщЬѕМўЯТКЯГЩАБЃЌЪЙЗгЬЊЪдвКБфКьЫљашвЊЕФЪБМфШчЯТЃК
ОнДЫЛиД№ЯТСаЮЪЬтЃК
(1)YаЮЙмзѓВрЙмжаЗЂЩњЗДгІЕФРызгЗНГЬЪН________________________ЁЃ
(2)ЬњЗлШідкЪЏУоШоЩЯЕФФПЕФЪЧ_________________________________
(3)ПЮЭтЛюЖЏаЁзщЕФЭЌбЇУЧШЯЮЊЃЌИУЪЕбщжаМДЪЙЗгЬЊБфКьвВВЛФмЫЕУїN2КЭH2ЗДгІКЯГЩСЫNH3ЃЌЕУГіДЫНсТлЕФРэгЩЪЧ________________________ЁЃ
ЧыФуСэЩшМЦвЛИіМђЕЅЕФЪЕбщбщжЄФуЕФРэгЩ____________________ЁЃгћНтОіетвЛЮЪЬтЃЌПЩвдбЁгУЯТЭМжаЕФ________зАжУСЌНгдкдзАжУжаЕФ________КЭ________жЎМфЁЃ

(4)дкЩЯЪіЪЕбщЙ§ГЬжаЃЌЮЊОЁПьЙлВьЕНЗгЬЊЪдвКБфКьЕФЪЕбщЯжЯѓЃЌгІИУПижЦN2КЭH2ЕФЬхЛ§БШЮЊ________БШНЯЪЪвЫЃЛЕЋИУзАжУЛЙФбвдЪЕЯжДЫФПЕФЃЌдвђЪЧ______________________________________ЁЃ
(5)ЪЕбщЙ§ГЬжаЭЈШыЪдЙмCжаЕФЦјЬхГЩЗжга________ЁЃ

ФГПЮЭтЛюЖЏаЁзщЭЈЙ§ВщдФзЪСЯКЭЖрДЮЪЕбщЃЌЕУЕНСЫШчЯТаХЯЂЃК
аХЯЂвЛЃКNaNO2ЙЬЬхКЭБЅКЭNH4ClШмвКЛьКЯМгШШЕФЙ§ГЬжаЗЂЩњШчЯТЗДгІЃК
ЂйNaNO2ЃЋNH4Cl
 NH4NO2ЃЋNaCl
NH4NO2ЃЋNaClЂкNH4NO2
 NH3ЁќЃЋHNO2
NH3ЁќЃЋHNO2Ђл2HNO2
 N2O3ЁќЃЋH2O
N2O3ЁќЃЋH2OЂм2NH3ЃЋN2O3
 2N2ЃЋ3H2O
2N2ЃЋ3H2OаХЯЂЖўЃКВщдФзЪСЯЃЌВЛЭЌЬхЛ§БШЕФN2ЁЂH2ЛьКЯЦјЬхдкЯрЭЌЪЕбщЬѕМўЯТКЯГЩАБЃЌЪЙЗгЬЊЪдвКБфКьЫљашвЊЕФЪБМфШчЯТЃК
| N2КЭH2ЕФЬхЛ§БШ | 5ЁУ1 | 3ЁУ1 | 1ЁУ1 | 1ЁУ3 | 1ЁУ5 |
| ЗгЬЊБфКьЩЋЫљашЪБМф/min | 8ЁЋ9 | 7ЁЋ8 | 6ЁЋ7 | 3ЁЋ4 | 9ЁЋ10 |
ОнДЫЛиД№ЯТСаЮЪЬтЃК
(1)YаЮЙмзѓВрЙмжаЗЂЩњЗДгІЕФРызгЗНГЬЪН________________________ЁЃ
(2)ЬњЗлШідкЪЏУоШоЩЯЕФФПЕФЪЧ_________________________________
(3)ПЮЭтЛюЖЏаЁзщЕФЭЌбЇУЧШЯЮЊЃЌИУЪЕбщжаМДЪЙЗгЬЊБфКьвВВЛФмЫЕУїN2КЭH2ЗДгІКЯГЩСЫNH3ЃЌЕУГіДЫНсТлЕФРэгЩЪЧ________________________ЁЃ
ЧыФуСэЩшМЦвЛИіМђЕЅЕФЪЕбщбщжЄФуЕФРэгЩ____________________ЁЃгћНтОіетвЛЮЪЬтЃЌПЩвдбЁгУЯТЭМжаЕФ________зАжУСЌНгдкдзАжУжаЕФ________КЭ________жЎМфЁЃ

(4)дкЩЯЪіЪЕбщЙ§ГЬжаЃЌЮЊОЁПьЙлВьЕНЗгЬЊЪдвКБфКьЕФЪЕбщЯжЯѓЃЌгІИУПижЦN2КЭH2ЕФЬхЛ§БШЮЊ________БШНЯЪЪвЫЃЛЕЋИУзАжУЛЙФбвдЪЕЯжДЫФПЕФЃЌдвђЪЧ______________________________________ЁЃ
(5)ЪЕбщЙ§ГЬжаЭЈШыЪдЙмCжаЕФЦјЬхГЩЗжга________ЁЃ
(1)ZnЃЋ2HЃЋ=Zn2ЃЋЃЋH2Ёќ
(2)діДѓЛьКЯЦјЬхгыДпЛЏМСЕФНгДЅУцЛ§ЃЌЪЙЗДгІНјааЕУИќПь
(3)ДгЗжВНЗДгІПЩжЊЃЌВњЩњN2ЕФЙ§ГЬжаЃЌгаПЩФмжБНгВњЩњАБЦјЁЁНЋЛьКЯМгШШВњЩњЕФЦјЬхжБНгЭЈШыЗгЬЊЪдвКЃЌШєЪдвКБфКьЃЌдђЫЕУїРэгЩГЩСЂЃЛЗёдђЃЌЫЕУїРэгЩВЛГЩСЂЁЁЂлЁЁAЁЁB
(4)1ЁУ3ЁЁЮоЗЈПижЦЭЈШыBжаN2КЭH2ЕФЬхЛ§БШ
(5)NH3ЁЂN2ЁЂH2
(2)діДѓЛьКЯЦјЬхгыДпЛЏМСЕФНгДЅУцЛ§ЃЌЪЙЗДгІНјааЕУИќПь
(3)ДгЗжВНЗДгІПЩжЊЃЌВњЩњN2ЕФЙ§ГЬжаЃЌгаПЩФмжБНгВњЩњАБЦјЁЁНЋЛьКЯМгШШВњЩњЕФЦјЬхжБНгЭЈШыЗгЬЊЪдвКЃЌШєЪдвКБфКьЃЌдђЫЕУїРэгЩГЩСЂЃЛЗёдђЃЌЫЕУїРэгЩВЛГЩСЂЁЁЂлЁЁAЁЁB
(4)1ЁУ3ЁЁЮоЗЈПижЦЭЈШыBжаN2КЭH2ЕФЬхЛ§БШ
(5)NH3ЁЂN2ЁЂH2
(1)ИљОнYаЮЙмгвВрЙмашМгШШЃЌЫЕУїгвВрЙмЗДгІжЦШЁN2ЃЌзѓВрЙмЗДгІжЦШЁH2ЁЃ(2)ЬњЗлШідкЪЏУоШоЩЯЕФФПЕФЪЧдіДѓгыЛьКЯЦјЬхЕФНгДЅУцЛ§ЃЌДгЖјЬсИпДпЛЏаЇТЪЃЌдіДѓЗДгІЫйТЪЁЃ(3)вђЮЊNH4NO2ЗжНтПЩВњЩњNH3ЃЌЫљвдВЛФмжЄУїN2КЭH2ЗДгІЩњГЩСЫNH3ЁЃжБНгНЋYаЮЙмжаЛьКЯЦјЬхЭЈШыЗгЬЊЪдвКЃЌШєЪдвКБфКьЃЌдђРэгЩГЩСЂЃЌЗёдђРэгЩВЛГЩСЂЁЃдкAЁЂBжЎМфМгвЛИіГ§NH3ЕФзАжУЃЌХХГ§СЫNH3ЕФИЩШХЁЃ(4)гЩБэПЩжЊV(N2)ЁУV(H2)ЃН1ЁУ3ЪБЃЌЗДгІзюПьЃЌдкYаЮЙмжаЃЌЮоЗЈПижЦЦјЬхЕФЬхЛ§ЁЃ(5)ЦјЬхГЩЗжжагаЩњГЩЕФNH3ЃЌЭЌЪБЛЙгаЮДЗДгІЕФN2ЁЂH2ЁЃ

СЗЯАВсЯЕСаД№АИ
 аТЫМЮЌМйЦкзївЕЪюМйМЊСжДѓбЇГіАцЩчЯЕСаД№АИ
аТЫМЮЌМйЦкзївЕЪюМйМЊСжДѓбЇГіАцЩчЯЕСаД№АИ РЖЬьНЬг§ЪюМйгХЛЏбЇЯАЯЕСаД№АИ
РЖЬьНЬг§ЪюМйгХЛЏбЇЯАЯЕСаД№АИ
ЯрЙиЬтФП
 g
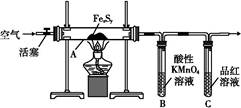
g 2CrO42ЃЃЈЛЦЩЋЃЉЃЋ2H+
2CrO42ЃЃЈЛЦЩЋЃЉЃЋ2H+

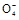 +2H2O+5SO2
+2H2O+5SO2 2Mn2++5S
2Mn2++5S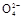 +4H+
+4H+

 АзЩЋГСЕэ
АзЩЋГСЕэ ГСЕэВЛШмНт
ГСЕэВЛШмНт ЦјЬх
ЦјЬх ЭЪЩЋ
ЭЪЩЋ
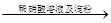 ШмвКБфРЖЩЋ
ШмвКБфРЖЩЋ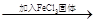 КьЩЋБфЩю
КьЩЋБфЩю