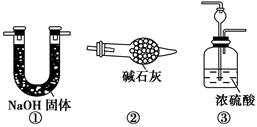
��Ŀ����
��13�֣�
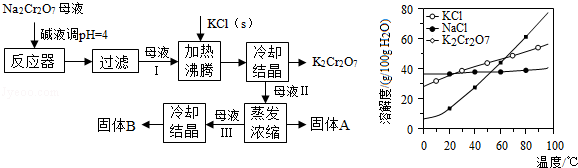
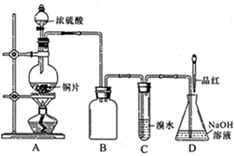
����ij������Һ�ۺ��ϵ�Ũ�ȵ�Na2Cr2O7��Fe2(SO4)3���Ʊ�K2Cr2O7��
�������£�
����NaOH��Һ��pH��3.6���������ɫ���������ˣ�
������Һ�м���Na2SO3��һ������������Na2SO4��
�������Na2SO4�����Һ��pHԼΪ5���õ�Cr(OH)3������
������KOH���������£���Cr(OH)3�м�������H2O2��Һ���õ���ɫ��Һ��
�������ɫ��Һ�м�������A����Һ��Ϊ�Ⱥ�ɫ��һ��������õ�K2Cr2O7���壻
�����ⶨK2Cr2O7����Ĵ��ȡ�
��֪��Cr2O72�����Ⱥ�ɫ����H2O 2CrO42������ɫ����2H+
2CrO42������ɫ����2H+
��1��������к��ɫ�����Ļ�ѧʽ�� ��
��2��������м���Na2SO3��Ŀ���� ��
��3��������з�Ӧ�����ӷ���ʽ�� ��
��4��������м��������A������ ��������ţ�
a��KOH b��K2CO3 c��H2SO4 d��SO2
��5��������IJ����ǣ�ȡ0.45 g K2Cr2O7��Ʒ�����Һ���ữ�����18.00 mL
0.50 mol/L��FeSO4��Һ��ǡ��ʹCr2O72����ȫת��ΪCr3+����Ʒ��K2Cr2O7�Ĵ����� ����ע��K2Cr2O7��Ħ������Ϊ294 g/mol��
��6����Ⱥ�ɫ��K2Cr2O7��Һ�У��μ�Ba(NO3)2��Һ��������ɫ��������ҺpH��С�����Ʋ��ɫ������ ����ҺpH��С��ԭ���� ��
����ij������Һ�ۺ��ϵ�Ũ�ȵ�Na2Cr2O7��Fe2(SO4)3���Ʊ�K2Cr2O7��
�������£�
����NaOH��Һ��pH��3.6���������ɫ���������ˣ�
������Һ�м���Na2SO3��һ������������Na2SO4��
�������Na2SO4�����Һ��pHԼΪ5���õ�Cr(OH)3������
������KOH���������£���Cr(OH)3�м�������H2O2��Һ���õ���ɫ��Һ��
�������ɫ��Һ�м�������A����Һ��Ϊ�Ⱥ�ɫ��һ��������õ�K2Cr2O7���壻
�����ⶨK2Cr2O7����Ĵ��ȡ�
��֪��Cr2O72�����Ⱥ�ɫ����H2O
 2CrO42������ɫ����2H+
2CrO42������ɫ����2H+��1��������к��ɫ�����Ļ�ѧʽ�� ��
��2��������м���Na2SO3��Ŀ���� ��
��3��������з�Ӧ�����ӷ���ʽ�� ��
��4��������м��������A������ ��������ţ�
a��KOH b��K2CO3 c��H2SO4 d��SO2
��5��������IJ����ǣ�ȡ0.45 g K2Cr2O7��Ʒ�����Һ���ữ�����18.00 mL
0.50 mol/L��FeSO4��Һ��ǡ��ʹCr2O72����ȫת��ΪCr3+����Ʒ��K2Cr2O7�Ĵ����� ����ע��K2Cr2O7��Ħ������Ϊ294 g/mol��
��6����Ⱥ�ɫ��K2Cr2O7��Һ�У��μ�Ba(NO3)2��Һ��������ɫ��������ҺpH��С�����Ʋ��ɫ������ ����ҺpH��С��ԭ���� ��
��13�֣�
��1��Fe(OH)3 ��1�֣�
��2����+6�۵�Cr��ԭΪ+3�� ��2�֣�
��3��2Cr(OH)3��3H2O2��4OH-��2CrO42-��8H2O ��2�֣�
��4��c ��2�֣���
��5��98% ��2�֣�
��6��BaCrO4 ��2�֣���
K2Cr2O7��Һ�д���ƽ�⣺Cr2O72�����Ⱥ�ɫ��+H2O 2CrO42������ɫ��+2H+������Ba(NO3)2��Һ����BaCrO4������c (CrO42��)���ͣ�ƽ�������ƶ���c (H+)����2�֣�
2CrO42������ɫ��+2H+������Ba(NO3)2��Һ����BaCrO4������c (CrO42��)���ͣ�ƽ�������ƶ���c (H+)����2�֣�
��1��Fe(OH)3 ��1�֣�
��2����+6�۵�Cr��ԭΪ+3�� ��2�֣�
��3��2Cr(OH)3��3H2O2��4OH-��2CrO42-��8H2O ��2�֣�
��4��c ��2�֣���
��5��98% ��2�֣�
��6��BaCrO4 ��2�֣���
K2Cr2O7��Һ�д���ƽ�⣺Cr2O72�����Ⱥ�ɫ��+H2O
 2CrO42������ɫ��+2H+������Ba(NO3)2��Һ����BaCrO4������c (CrO42��)���ͣ�ƽ�������ƶ���c (H+)����2�֣�
2CrO42������ɫ��+2H+������Ba(NO3)2��Һ����BaCrO4������c (CrO42��)���ͣ�ƽ�������ƶ���c (H+)����2�֣������������1��������к��ɫ������������������ѧʽ��Fe(OH)3��
��2�����������Cr(OH)3�������ɣ�˵��֮ǰ����Һ�д���Cr3+�����Լ���Na2SO3��Ŀ���ǽ�+6�۵�Cr��ԭΪ+3�ۣ�
��3��������֪�û�ɫ��2CrO42������ɫ�����Բ�����з�����������ԭ��Ӧ����Ӧ�����ӷ���ʽ��2Cr(OH)3��3H2O2��4OH-��2CrO42-��8H2O��
��4������Cr2O72�����Ⱥ�ɫ����H2O
 2CrO42������ɫ����2H+��֪�������A���ʿ�ʹƽ�������ƶ�������Ӧ���������ʣ��ų�ab������������л�ԭ�ԣ�����CrO42����Ӧ������Cr3+���������CrO42����Ӧ������ʹƽ�������ƶ�����ѡc��
2CrO42������ɫ����2H+��֪�������A���ʿ�ʹƽ�������ƶ�������Ӧ���������ʣ��ų�ab������������л�ԭ�ԣ�����CrO42����Ӧ������Cr3+���������CrO42����Ӧ������ʹƽ�������ƶ�����ѡc����5��FeSO4�����ʵ�����0.018L��0.5mol/L=0.009mol����������Fe3+ʧ���ӵ����ʵ�����0.009mol����
K2Cr2O7�����ʵ�����x����ԭ��Cr3+���õ��ӵ����ʵ�����2x��3�����ݵ�ʧ�����غ㣬��
2x��3=0.009mol������x=0.0015mol�����Ʒ��K2Cr2O7�Ĵ�����0.0015mol��294g/mol��0.45g��100%=98%;
��6�������ӵ���ɫ�жϸû�ɫ������BaCrO4 ����Һ�д���ƽ�⣺Cr2O72�����Ⱥ�ɫ��+H2O
 2CrO42������ɫ��+2H+������Ba(NO3)2��Һ����BaCrO4������ʹc (CrO42��)���ͣ�ƽ�������ƶ���c (H+)����pH���͡�
2CrO42������ɫ��+2H+������Ba(NO3)2��Һ����BaCrO4������ʹc (CrO42��)���ͣ�ƽ�������ƶ���c (H+)����pH���͡�
��ϰ��ϵ�д�
 ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
�����Ŀ

 ��3����֪C����0��1 mol Cl2�μӷ�Ӧ��������һ����������֪C�з�Ӧ�Ļ�ѧ����ʽΪ ��
��3����֪C����0��1 mol Cl2�μӷ�Ӧ��������һ����������֪C�з�Ӧ�Ļ�ѧ����ʽΪ ��


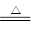 NH4NO2��NaCl
NH4NO2��NaCl