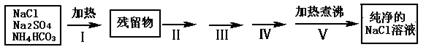
��Ŀ����
�����йز�����˵������ȷ����( )
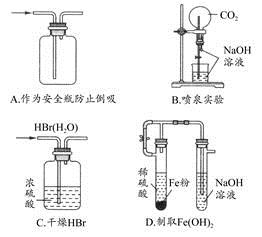
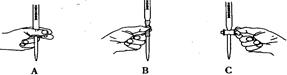
| A�����к͵ζ���ʵ���У�����ƿ����ƿ������ˮϴ����ʹ�ã��ζ��ܺ���Һ��������ˮϴ������������ϴ��ʹ�� |
| B����ȥ����CO2�л��е�����SO2���ɽ������������ͨ��ʢ������KMnO4��Һ��Ũ�����ϴ��ƿ |
| C��������þ��Һ�м����������ƿ��Եõ�������þ���� |
| D����Mg(OH)2����ת���������У�������ϡ���ᣬ�������ɵ���ˮMgCl2���� |
D
�ζ��ܺ���Һ�ܲ�����ˮ��ϡ��Һ�壬��������ϴ��ʹ�ã�A��ȷ������SO2�Ļ�ԭ�ԣ�������KMnO4��Һ��ȥ,����Ũ�����ȥ��������ˮ������B��ȷ��NaOH��MgSO4�������ֽⷴӦ����Mg(OH)2��������C��ȷ����������MgCl2��Һ����MgCl2��ˮ�⣬���յõ�Mg(OH)2����D����ȷ��

��ϰ��ϵ�д�
�����Ŀ