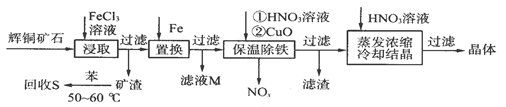
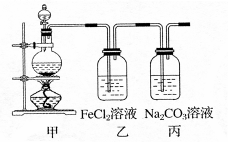
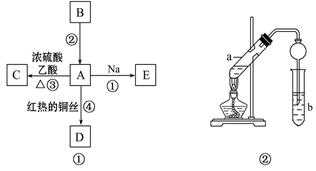
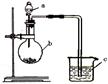
ΧβΡΩΡΎ»ί
Β―ι “–η“Σ¥ΩΨΜΒΡNaCl»ή“ΚΘ§ΒΪ Β―ι “ΒΡNaClΨßΧεΜλ”–…ΌΝΩNa2SO4ΚΆNH4HCO3Θ§Ρ≥Ά§―ßΑ¥»γœ¬Νς≥ΧΆΦ…ηΦΤ Β―ι≥ΐ»Ξ‘”÷ Θ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
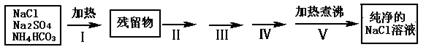
Θ®1Θ©≤Ϋ÷ηI≥ΐ»ΞΒΡ‘”÷ «Θ®ΧνΜ·―ß ΫΘ©_______________Θ§÷±Ϋ”Φ”»»“ΣΚΟ”ΎΦ”«ΩΦνΚσ‘ΌΫχ––Φ”»»Θ§άμ”… « ΘΜ
Θ®2Θ©Α¥Νς≥ΧΆΦΆξ≥… Β―ι…ηΦΤΘ§ΫΪœύΙΊΒΡ Β―ι≤ΌΉςΓΔ Β―ιœ÷œσΚΆ Β―ιΡΩΒΡΧν–¥‘Ύœ¬±μ÷–ΘΚ
Θ®3Θ©»τΉνΚσΒΟΒΫ20ΓφΒΡNaCl±ΞΚΆ»ή“ΚΓΘ“―÷Σ20Γφ ±NaClΒΡ»ήΫβΕ»ΈΣ36.0gΓΔNaCl±ΞΚΆ»ή“ΚΒΡΟήΕ»ΈΣ1.12g/cm3 Θ§‘ρ20ΓφΒΡNaCl±ΞΚΆ»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ mol/LΘ®ΦΤΥψΫαΙϊ±ΘΝτ»ΐΈΜ”––ß ΐΉ÷Θ©ΓΘ

Θ®1Θ©≤Ϋ÷ηI≥ΐ»ΞΒΡ‘”÷ «Θ®ΧνΜ·―ß ΫΘ©_______________Θ§÷±Ϋ”Φ”»»“ΣΚΟ”ΎΦ”«ΩΦνΚσ‘ΌΫχ––Φ”»»Θ§άμ”… « ΘΜ
Θ®2Θ©Α¥Νς≥ΧΆΦΆξ≥… Β―ι…ηΦΤΘ§ΫΪœύΙΊΒΡ Β―ι≤ΌΉςΓΔ Β―ιœ÷œσΚΆ Β―ιΡΩΒΡΧν–¥‘Ύœ¬±μ÷–ΘΚ
| ≤ΌΉς≤Ϋ÷η | Β―ιœ÷œσ | Β―ιΡΩΒΡ |
| ≤Ϋ÷ηIIΘΚΫΪ≤–ΝτΈο»ήΫβΒΟΒΫ»ή“ΚΘ§ | | |
| ≤Ϋ÷ηIIIΘΚ |  | |
| ≤Ϋ÷ηIVΘΚΙΐ¬ΥΘ§Άυ¬Υ“Κ÷– | | |
| ≤Ϋ÷ηVΘΚΫΪ»ή“ΚΦ”»»÷σΖ– |  | |
Θ®3Θ©»τΉνΚσΒΟΒΫ20ΓφΒΡNaCl±ΞΚΆ»ή“ΚΓΘ“―÷Σ20Γφ ±NaClΒΡ»ήΫβΕ»ΈΣ36.0gΓΔNaCl±ΞΚΆ»ή“ΚΒΡΟήΕ»ΈΣ1.12g/cm3 Θ§‘ρ20ΓφΒΡNaCl±ΞΚΆ»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ mol/LΘ®ΦΤΥψΫαΙϊ±ΘΝτ»ΐΈΜ”––ß ΐΉ÷Θ©ΓΘ
Θ®17Ζ÷Θ©
Θ®1Θ©NH4HCO3Θ®2Ζ÷Θ©
≤ΌΉςΦρΒΞΘΜΫΎ Γ ‘ΦΝΘΜ≤Μ“ΐ»κ–¬ΒΡ‘”÷ Θ®2Ζ÷Θ§¥πΤδ÷–ΝΫΒψΩ…ΒΟ¬ζΖ÷Θ©
Θ®2Θ©Θ®Ι≤10Ζ÷Θ§≥ΐΉΔΟςΆβΟΩΩ’1Ζ÷Θ§Ή≈÷ΊΚ≈ΈΣΗχΖ÷ΒψΘ©
Θ®3Θ©5.07 Θ®3Ζ÷Θ©
Θ®1Θ©NH4HCO3Θ®2Ζ÷Θ©
≤ΌΉςΦρΒΞΘΜΫΎ Γ ‘ΦΝΘΜ≤Μ“ΐ»κ–¬ΒΡ‘”÷ Θ®2Ζ÷Θ§¥πΤδ÷–ΝΫΒψΩ…ΒΟ¬ζΖ÷Θ©
Θ®2Θ©Θ®Ι≤10Ζ÷Θ§≥ΐΉΔΟςΆβΟΩΩ’1Ζ÷Θ§Ή≈÷ΊΚ≈ΈΣΗχΖ÷ΒψΘ©
| ≤ΌΉς≤Ϋ÷η | Β―ιœ÷œσ | Β―ιΡΩΒΡ |
| ≤Ϋ÷ηIIΘΚΆυ»ή“Κ÷–ΒΈΦ”ΙΐΝΩΒΡBaCl2»ή“Κ | …ζ≥…ΑΉ…Ϊ≥ΝΒμ | ≥ΐ»ΞSO42Θ≠άκΉ” |
| ≤Ϋ÷ηIIIΘΚΆυ–ϋΉ«“Κ÷–Θ®ΜρΙΐ¬ΥΚσΘ§Άυ¬Υ“Κ÷–Θ©ΒΈΦ”ΙΐΝΩΒΡNa2CO3»ή“Κ |  | ≥ΐ»ΞΙΐΝΩΒΡBa2+άκΉ” |
| ≤Ϋ÷ηIVΘΚΒΈΦ” ΝΩΒΡ―ΈΥα | ≤ζ…ζ…ΌΝΩΤχ≈ί | ≥ΐ»ΞΙΐΝΩΒΡCO32Θ≠άκΉ” |
  | | «ΐΗœ»ήΫβ‘Ύ»ή“Κ÷–ΒΡCO2ΚΆHClΤχΧεΘ®2Ζ÷Θ© |
Θ®3Θ©5.07 Θ®3Ζ÷Θ©
‘ΧβΖ÷ΈωΘΚΘ®1Θ©οß―Έ≤ΜΈ»Ε®Θ§ ή»»“ΉΖ÷ΫβΘ§‘ρΦ”»»ΒΡΡΩΒΡ «≥ΐ»ΞΙΧΧεΜλΚœΈο÷–ΒΡΧΦΥα«βοßΘ§NH4HCO3
 NH3Γϋ+H2OΓϋ+CO2ΓϋΘΜ«ΩΦν”κοß―Έ»ί“ΉΖ¥”Π…ζ≥…―ΈΓΔΑ±ΤχΚΆΥ°Θ§≥ΐ»ΞΨ…‘”÷ ΒΡΆ§ ±Θ§”÷“ΐ»κ–¬‘”÷ Θ§«“‘ω¥σ ‘ΦΝΓΔ≤ΌΉς≤Ϋ÷η‘ωΕύΘΜΘ®2Θ©≥ΐ»ΞΧΦΥα«βοßΚσΘ§œ»ΫΪΥυΒΟΑΉ…ΪΙΧΧε»ήΫβΘ§‘Όœρ»ή“Κ÷–Φ”»κΙΐΝΩBaCl2»ή“ΚΘ§ ΙΝρΥαΡΤΆξ»ΪΖ¥”ΠΘ§…ζ≥…ΑΉ…ΪΒΡΝρΥα±Β≥ΝΒμΚΆNaClΘ§≥ΐ»Ξ»ή“Κ÷–ΒΡΝρΥαΗυάκΉ”Θ§ΒΪ «“ΐ»κΒΡ±ΒάκΉ” «–¬ΒΡ‘”÷ ΘΜΈΣΝΥ≥ΐ»Ξ±ΒάκΉ”Θ§”Πœρ–ϋΉ«“ΚΜρΙΐ¬ΥΥυΒΟ¬Υ“Κ÷–Φ”»κΙΐΝΩΧΦΥαΡΤ»ή“ΚΘ§ Ι¬»Μ·±ΒΆξ»Ϊ”κΧΦΥαΡΤΖ¥”ΠΘ§…ζ≥…ΑΉ…ΪΒΡΧΦΥα±Β≥ΝΒμΚΆ¬»Μ·ΡΤΘ§≥ΐ»Ξ»ή“Κ÷–ΒΡ±ΒάκΉ”Θ§ΒΪ «“ΐ»κΒΡΧΦΥαΗυάκΉ” «–¬ΒΡ‘”÷ ΘΜΙΐ¬ΥΘ§Ζ÷άκ≥ωΝρΥα±ΒΓΔΧΦΥα±Β≥ΝΒμ÷°ΚσΘ§ΈΣΝΥ≥ΐ»Ξ¬Υ“Κ÷–ΒΡΧΦΥαΗυάκΉ”Θ§”ΠœρΤδ÷–Φ”»κ ΝΩ―ΈΥαΘ§ΧΦΥαΡΤ”κ―ΈΥαΖ¥”ΠΘ§…ζ≥…¬»Μ·ΡΤΓΔΕΰ―θΜ·ΧΦΤχΧεΚΆΥ°Θ§≥ΐ»Ξ»ή“Κ÷–ΒΡΧΦΥαΗυάκΉ”Θ§ΒΪ «“ΐ»κΒΡ―ΈΥα «–¬ΒΡ‘”÷ ΘΜ―ΈΥαΚΆΥ°ΒΡΖ–ΒψΫœΒΆΘ§¬»Μ·ΡΤΒΡΖ–ΒψΗΏΘ§ΫΪ»ή“Κ÷σΖ–Θ§Ω…“‘≥ΐ»Ξ»ή“Κ÷–ΒΡ¬»Μ·«βΓΔΕΰ―θΜ·ΧΦΚΆΥ°Θ§’τΖΔΩ…ΒΟ¥ΩΨΜΒΡ¬»Μ·ΡΤΙΧΧεΘΜΘ®3Θ©“άΧβ“βΘ§36.0gNaCl»ή”Ύ100gΥ°÷–Θ§Φ¥Ω…≈δ÷Τ20ΓφΒΡ±ΞΚΆNaCl»ή“ΚΘΜ”…”Ύ»ή“ΚΒΡ÷ ΝΩΒ»”Ύ»ή÷ ÷ ΝΩ”κ»ήΦΝ÷ ΝΩ÷°ΚΆΘ§‘ρ36.0gNaCl»ή”Ύ100gΥ°–Έ≥…136.0g±ΞΚΆNaCl»ή“ΚΘΜ”…”Ύ»ή“ΚΧεΜΐΒ»”Ύ»ή“Κ÷ ΝΩ”κ»ή“ΚΟήΕ»ΒΡ±»÷ΒΘ§‘ρ±ΞΚΆNaCl»ή“ΚΒΡΧεΜΐΈΣ
NH3Γϋ+H2OΓϋ+CO2ΓϋΘΜ«ΩΦν”κοß―Έ»ί“ΉΖ¥”Π…ζ≥…―ΈΓΔΑ±ΤχΚΆΥ°Θ§≥ΐ»ΞΨ…‘”÷ ΒΡΆ§ ±Θ§”÷“ΐ»κ–¬‘”÷ Θ§«“‘ω¥σ ‘ΦΝΓΔ≤ΌΉς≤Ϋ÷η‘ωΕύΘΜΘ®2Θ©≥ΐ»ΞΧΦΥα«βοßΚσΘ§œ»ΫΪΥυΒΟΑΉ…ΪΙΧΧε»ήΫβΘ§‘Όœρ»ή“Κ÷–Φ”»κΙΐΝΩBaCl2»ή“ΚΘ§ ΙΝρΥαΡΤΆξ»ΪΖ¥”ΠΘ§…ζ≥…ΑΉ…ΪΒΡΝρΥα±Β≥ΝΒμΚΆNaClΘ§≥ΐ»Ξ»ή“Κ÷–ΒΡΝρΥαΗυάκΉ”Θ§ΒΪ «“ΐ»κΒΡ±ΒάκΉ” «–¬ΒΡ‘”÷ ΘΜΈΣΝΥ≥ΐ»Ξ±ΒάκΉ”Θ§”Πœρ–ϋΉ«“ΚΜρΙΐ¬ΥΥυΒΟ¬Υ“Κ÷–Φ”»κΙΐΝΩΧΦΥαΡΤ»ή“ΚΘ§ Ι¬»Μ·±ΒΆξ»Ϊ”κΧΦΥαΡΤΖ¥”ΠΘ§…ζ≥…ΑΉ…ΪΒΡΧΦΥα±Β≥ΝΒμΚΆ¬»Μ·ΡΤΘ§≥ΐ»Ξ»ή“Κ÷–ΒΡ±ΒάκΉ”Θ§ΒΪ «“ΐ»κΒΡΧΦΥαΗυάκΉ” «–¬ΒΡ‘”÷ ΘΜΙΐ¬ΥΘ§Ζ÷άκ≥ωΝρΥα±ΒΓΔΧΦΥα±Β≥ΝΒμ÷°ΚσΘ§ΈΣΝΥ≥ΐ»Ξ¬Υ“Κ÷–ΒΡΧΦΥαΗυάκΉ”Θ§”ΠœρΤδ÷–Φ”»κ ΝΩ―ΈΥαΘ§ΧΦΥαΡΤ”κ―ΈΥαΖ¥”ΠΘ§…ζ≥…¬»Μ·ΡΤΓΔΕΰ―θΜ·ΧΦΤχΧεΚΆΥ°Θ§≥ΐ»Ξ»ή“Κ÷–ΒΡΧΦΥαΗυάκΉ”Θ§ΒΪ «“ΐ»κΒΡ―ΈΥα «–¬ΒΡ‘”÷ ΘΜ―ΈΥαΚΆΥ°ΒΡΖ–ΒψΫœΒΆΘ§¬»Μ·ΡΤΒΡΖ–ΒψΗΏΘ§ΫΪ»ή“Κ÷σΖ–Θ§Ω…“‘≥ΐ»Ξ»ή“Κ÷–ΒΡ¬»Μ·«βΓΔΕΰ―θΜ·ΧΦΚΆΥ°Θ§’τΖΔΩ…ΒΟ¥ΩΨΜΒΡ¬»Μ·ΡΤΙΧΧεΘΜΘ®3Θ©“άΧβ“βΘ§36.0gNaCl»ή”Ύ100gΥ°÷–Θ§Φ¥Ω…≈δ÷Τ20ΓφΒΡ±ΞΚΆNaCl»ή“ΚΘΜ”…”Ύ»ή“ΚΒΡ÷ ΝΩΒ»”Ύ»ή÷ ÷ ΝΩ”κ»ήΦΝ÷ ΝΩ÷°ΚΆΘ§‘ρ36.0gNaCl»ή”Ύ100gΥ°–Έ≥…136.0g±ΞΚΆNaCl»ή“ΚΘΜ”…”Ύ»ή“ΚΧεΜΐΒ»”Ύ»ή“Κ÷ ΝΩ”κ»ή“ΚΟήΕ»ΒΡ±»÷ΒΘ§‘ρ±ΞΚΆNaCl»ή“ΚΒΡΧεΜΐΈΣ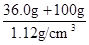 =
=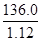 cm3=
cm3=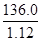 mLΘΜ»ή÷ ΒΡΈο÷ ΒΡΝΩΒ»”Ύ÷ ΝΩ”κΡΠΕϊ÷ ΝΩΒΡ±»÷ΒΘ§‘ρ136.0g±ΞΚΆNaCl»ή“Κ÷–n(NaCl)=
mLΘΜ»ή÷ ΒΡΈο÷ ΒΡΝΩΒ»”Ύ÷ ΝΩ”κΡΠΕϊ÷ ΝΩΒΡ±»÷ΒΘ§‘ρ136.0g±ΞΚΆNaCl»ή“Κ÷–n(NaCl)=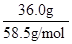 =
=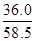 molΘΜ”…”Ύc=n/VΘ§‘ρ20ΓφΒΡ±ΞΚΆNaCl»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ
molΘΜ”…”Ύc=n/VΘ§‘ρ20ΓφΒΡ±ΞΚΆNaCl»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ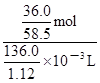 =
=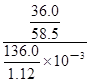 mol/LΓ÷5.07mol/LΓΘ
mol/LΓ÷5.07mol/LΓΘ
ΝΖœΑ≤αœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ