��Ŀ����
����Ŀ�������������ƣ�Na2S2O4�����м�ǿ�Ļ�ԭ�ԣ����ȡ���ˮ���ᷢ���ֽⷴӦ�ų��������ȣ���������ȼ�գ��������Ҵ�����������������ˮ��Һ���ȶ����ڡ�
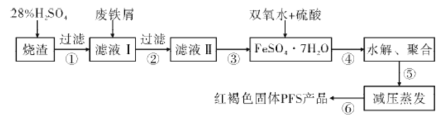
��1��п�۷����Ʊ�Na2S2O4��һ�ֳ�����������ԭ����ͼ1��ʾ��
���뽫п�ۺ�ˮ�Ƴ�����Һ��ԭ���� ______ ������Na2SO3�����ij�ᷴӦ�Ʊ�SO2���壬����Ϊ����������ѡ�õ����� ______
A��Ũ���� B����������Ϊ70%��H2SO4
C��ϡ���� D����������Ϊ10%��ϡ����
�ڲ���III���̽�Ϊ���ӣ������漰���ˡ�ϴ�ӡ�����Ȳ�������д��ϴ�ӹ��̵IJ��������� ______ ��
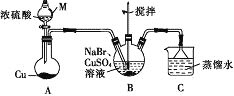
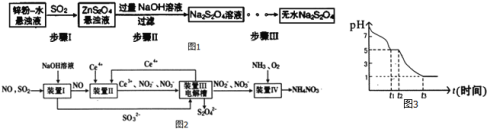
��2�����մ�����Ⱦ��SO2��NO�����Na2S2O4��NH4NO3��Ʒ������ͼ��ͼ2��CeΪ��Ԫ�أ���
��װ�â��У����������£�NO��Ce4+�����IJ�����Ҫ��NO3-��NO2-��д������NO3-�����ӷ���ʽ ______
��װ��III�е������������ĵ缫��ӦΪ ______ ��
����֪����װ��IV����Һ�У�NO2-��Ũ��Ϊa gL-1��Ҫʹ1m3����Һ�е�NO2-��ȫת��ΪNH4NO3����������װ��IV��ͨ���״���µ�O2 ______ L�����ú�a����ʽ��ʾ������������������
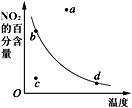
��3������С��ⶨ0.050molL-1Na2S2O4��Һ�ڿ�����pH�仯��ͼ3��0-t1����Ҫ����HSO3-����0-t1�������ӷ�Ӧ����ʽΪ ______ ��t1ʱ����Һ������Ũ���ɴ�С��˳���� ______ ��t3ʱ��Һ����Ҫ�����ӷ����� ______ ��
���𰸡�����п��Ӧ�ı�������ӿ컯ѧ��Ӧ���� B �����Ҵ�����û�������˸ɺ��ظ�����2-3�� NO+3Ce4++2H2O=NO3-+3Ce3++4H+ 2SO32-+2H2O+2e-=S2O42-+4OH- 243a 2S2O42-+O2+2H2O=4HSO3- c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-�� SO42-
��������
п�ۡ�ˮ�γɷ�ɢϵ��ͨ���������Ӧ�õ�ZnS2O4���������NaOH��Һ��Ӧ�õ�Na2S2O4��Һ���پ������ӵIJ������̵õ�Na2S2O4������
��1������п�ۺ�ˮ�Ƴ�����Һ��ԭ���ǣ�����п��Ӧ�ı�������ӿ컯ѧ��Ӧ���ʣ�A������ӷ����Ʊ��Ķ���������HCl��B�����������70%���ᷴӦ�Ƶö�������C��������ƾ��л�ԭ�ԣ�������������ԣ�����������ԭ��Ӧ�������Ƶö�������D���������������ˮ��10%������Ũ��̫С�������ڶ���������ݳ�����ѡB��
�ʴ�Ϊ������п��Ӧ�ı�������ӿ컯ѧ��Ӧ���ʣ�B��
�����������Ϣ�����Ҵ�ϴ�ӣ���ȥ���������ʣ����������ܽ�µ���ʧ��ϴ�ӹ��̵IJ��������������Ҵ�����û�������˸ɺ��ظ�����2-3�Σ�
�ʴ�Ϊ�������Ҵ�����û�������˸ɺ��ظ�����2-3�Σ�
��2����װ�����У����������£�NO��Ce4+��������NO3-����Ce4+����ԭΪCe3+���������ݵ�ʧ�����غ㡢ԭ���غ�͵���غ㣬��Ӧ���ӷ���ʽΪ��NO+3Ce4++2H2O=NO3-+3Ce3++4H+��
�ʴ�Ϊ��NO+3Ce4++2H2O=NO3-+3Ce3++4H+��
����������������ԭ��Ӧ��SO32-����ԭΪS2O42-���缫��ӦʽΪ��2SO32-+2H2O+2e-=S2O42-+4OH-��
�ʴ�Ϊ��2SO32-+2H2O+2e-=S2O42-+4OH-��
��NO2-��Ũ��ΪagL-1��Ҫʹ1m3����Һ�е�NO2-��ȫת��ΪNH4NO3����ʧȥ������Ŀ�ǣ�![]() mol��2�������ı���������������V����õ�������Ŀ�ǣ�
mol��2�������ı���������������V����õ�������Ŀ�ǣ�![]() nol��4�����ݵ����غ㣺
nol��4�����ݵ����غ㣺![]() mol��2=
mol��2=![]() nol��4�����V=243a��
nol��4�����V=243a��
�ʴ�Ϊ��243a��
��3��0��t1����Ҫ������HSO3-����Һ�����ԣ�˵��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�Na2S2O4��Һ�ڿ������ױ������������������ƣ�0��t1�������ӷ�Ӧ����ʽΪ��2S2O42-+O2+2H2O=4HSO3-������������ĵ���̶Ȳ���ˮҲ�������������ӣ���t1ʱ����Һ������Ũ���ɴ�С��˳���ǣ�c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
t3ʱ��Һ��pH=1��˵����Һ�����Խ�ǿ��������������Ʊ�����Ϊ�������ƣ�����Һ����Ҫ�����ӷ�����SO42-��
�ʴ�Ϊ��2S2O42-+O2+2H2O=4HSO3-��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����SO42-��