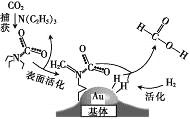
��Ŀ����
����Ŀ��ij��ȤС���ͬѧ���ʵ���Ʊ�CuBr(��ɫ�ᾧ�Է�ĩ������ˮ���������Ҵ����л��ܼ�)��ʵ��װ��(�г֡�����������)��ͼ��ʾ��
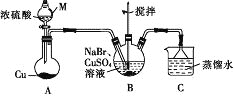
(1)����M��������________��
(2)����M�е�Ũ���ỻ��70%��H2SO4����Բ����ƿ�еĹ����Լ�Ϊ______(�ѧʽ)��
(3)B�з�����Ӧ�Ļ�ѧ����ʽΪ_______����˵��B�з�Ӧ����ɵ�������_____����B��Cu2+��δ��ȫ����ԭ�����˼�����Լ���_______(����)��
a.Һ�� b.Na2SO4 c.���� d.Na2S2O3
(4)���й��ڹ��˵���������ȷ����_______ (����)��
a.©��ĩ�˾�����Բ������ձ���
b.����ֽ��ʪ��ʹ�����©���ڱ�
c.��ֽ��Ե���Ը߳�©����
d.�ò�������©������������Լӿ��������
(5)ϴ��ʱ������װ��C�е�����Һ��ϴ����Ŀ����_______�����������ܽ�SO2���Ҵ�������ϴ�ӵ�Ŀ����________��
���𰸡���Һ©�� Na2SO3(��K2SO3��NaHSO3��KHSO3��) 2CuSO4+2NaBr+SO2+2H2O=2CuBr��+Na2SO4+2H2SO4 ��Һ��ɫ��ȥ d acd ��ֹCuBr������ ���Ҵ���ȥ��������ˮ�����ø��ӷ������ѳ�ȥ�Ҵ���ʹ����ٸ���
��������
(1)���������ṹ�ж����������ƣ�
(2)���ݸ��ֽⷴӦ�Ĺ���ѡ���Լ���
(3)��B��NaBr��SO2��CuSO4��H2O�ᷢ��������ԭ��Ӧ����CuBr���������õ����غ㡢ԭ���غ㣬��д��Ӧ�Ļ�ѧ����ʽ������Cu2+��ˮ��Һ����ɫ����ϸ÷�Ӧ���ص��жϷ�Ӧ��ȫ����������������ʣ�
(4)���ݹ��˲�����Ŀ�ġ�������ʹ�÷������
(5)CuBr���ױ�����������SO2��ˮ��Һ���л�ԭ�Է��������H2O���������Ҵ��У����Ҵ������Ѿ����ӷ������ʷ�����
(1)����װ��ͼ������M�Ľṹ��֪�������������Ƿ�Һ©����
(2)���ݸ��ֽⷴӦ�Ĺ��ɿ���70%��H2SO4��Na2SO3��K2SO3��NaHSO3��KHSO3�ȷ�Ӧ��ȡSO2���壬��Ӧ����ʽΪH2SO4+Na2SO3=Na2SO4+H2O+SO2����
(3)��B��BaBr��SO2��CuSO4��H2O�ᷢ��������ԭ��Ӧ����CuBr���������õ����غ㡢ԭ���غ㣬�ɵø÷�Ӧ�Ļ�ѧ����ʽΪ��2CuSO4+2NaBr+SO2+2H2O=2CuBr��+Na2SO4+2H2SO4��
Cu2+��ˮ��Һ����ɫ������Ӧ��ȫ������Һ�в��ٺ���Cu2+����Һ����ɫ��ȥ������CuSO4��ǿ�������Σ�����Һ��Cu2+ˮ��ʹ��Һ�����ԣ�H+��Na2S2O3��Ӧ����SO2���壬�Դ�ʹ��Ӧ�Ľ��У������B��Cu2+��δ��ȫ����ԭ�����˼�����Լ���Na2S2O3���ʺ���ѡ����d��
(4)a.©��ĩ�˾�������ձ��ڣ��Ϳ���ʹ���˵õ�����Һ���ձ��ڱڲ��Ͻ��뵽�ձ��У�a����
b.����ֽ��ʪ��ʹ�����©���ڱڣ��Ϳ���ʹ������ַ��룬b��ȷ��
c.Ϊ��ʹ�����ԵĹ�����Һ�����ʷ��룬��ֽ��ԵҪ����©���ڱ�Ե��c����
d.�ò�����������ʹ�������뵽�������У���©���в����ò����������������ʹ��ֽ���𣬵��²��ܹ��ˣ����ܷ������d����
�ʺ���ѡ����acd��
(5)SO2�Ǵ�����Ⱦ�Ϊ��ֹ����Ⱦ������������ˮ����SO2���õ�H2SO3��Һ�������ʾ��л�ԭ�ԣ���H2SO3��Һϴ�ӣ��Ϳ��Ա���CuBr�������е�����������Ȼ���������ܽ�SO2���Ҵ�������ϴ�ӵ�Ŀ�������Ҵ���ȥ��������ˮ�����ø��ӷ������ѳ�ȥ�Ҵ���ʹ����ٸ��

 ��У����ϵ�д�
��У����ϵ�д�