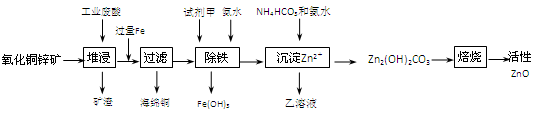
��Ŀ����
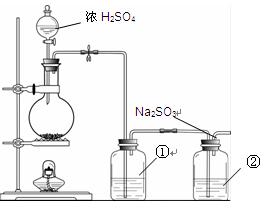
��13�֣�ij�о���ѧϰС���������ռ���������Ϣ��Fe(NO3)3��Һ����ʴ�������������������Ρ����Ƕ�ʴ������ԭ�����������̽����
��ʵ�顿���������Ʊ�����������Fe(NO3)3��Һ��Ӧ�����������ܽ⡣
��������衿
����1��Fe3�����������ԣ�������Ag��
����2��Fe(NO3)3��Һ�����ԣ��ڴ�����������NO3-������Ag��
�����ʵ�鷽������֤���衿
��1����ͬѧ������ʵ����������м����Fe2������֤�˼���1��������д��Fe3������Ag�����ӷ���ʽ�� ����ͬѧ��������ͬŨ��FeCl3��Fe(NO3)3�ܽ�������FeC l3��Һ�ܽ�Ag�ķ�Ӧ�������ȫ���������ԭ����������������������������
l3��Һ�ܽ�Ag�ķ�Ӧ�������ȫ���������ԭ����������������������������
��������������������������������������������������������������������������
��2����ͬѧ���ʵ����֤����2�����������±������ݣ���ʾ�� NO3-�ڲ�ͬ�����µĻ�ԭ����ϸ��ӣ���ʱ���Թ۲쵽�����������
��˼���뽻����
��3����ͬѧ��֤�˼���1����������ͬѧ��֤�˼���2Ҳ���������ͬѧ�ɴ˵ó����ۣ�Fe(NO3)3��Һ�е�Fe3����NO3����������Ag��
���Ƿ�ͬ���ͬѧ�Ľ��� �����������ɣ� ��
��ʵ�顿���������Ʊ�����������Fe(NO3)3��Һ��Ӧ�����������ܽ⡣
��������衿
����1��Fe3�����������ԣ�������Ag��
����2��Fe(NO3)3��Һ�����ԣ��ڴ�����������NO3-������Ag��
�����ʵ�鷽������֤���衿
��1����ͬѧ������ʵ����������м����Fe2������֤�˼���1��������д��Fe3������Ag�����ӷ���ʽ�� ����ͬѧ��������ͬŨ��FeCl3��Fe(NO3)3�ܽ�������FeC
 l3��Һ�ܽ�Ag�ķ�Ӧ�������ȫ���������ԭ����������������������������
l3��Һ�ܽ�Ag�ķ�Ӧ�������ȫ���������ԭ������������������������������������������������������������������������������������������������������
��2����ͬѧ���ʵ����֤����2�����������±������ݣ���ʾ�� NO3-�ڲ�ͬ�����µĻ�ԭ����ϸ��ӣ���ʱ���Թ۲쵽�����������
| ʵ�鲽�裨��Ҫ��д����������̣� | Ԥ������ͽ��� |
| �� �� ���� | ��������ʧ������2������ ����������ʧ������2�������� |
��3����ͬѧ��֤�˼���1����������ͬѧ��֤�˼���2Ҳ���������ͬѧ�ɴ˵ó����ۣ�Fe(NO3)3��Һ�е�Fe3����NO3����������Ag��
���Ƿ�ͬ���ͬѧ�Ľ��� �����������ɣ� ��
��1��Fe3++Ag= Fe2++Ag+��2�֣� FeCl3��Cl����Ag������AgCl���ٽ�����Ӧ��ȫ����2�֣�
��2���ٲⶨ����ʵ���õ�Fe(NO3)3��Һ��pH����2�֣�
��2���ٲⶨ����ʵ���õ�Fe(NO3)3��Һ��pH����2�֣�

��

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ
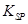 = 2��70��10-39]
= 2��70��10-39]

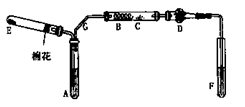
 l2����Ư���ԡ�
l2����Ư���ԡ�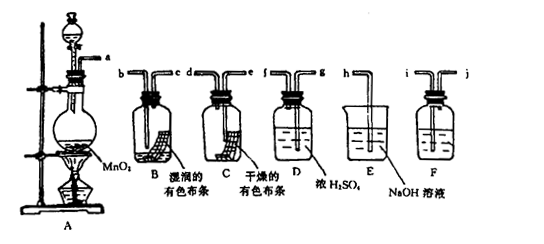
 ��
�� ��ɫ����������ɵó��Ľ�����
��ɫ����������ɵó��Ľ����� 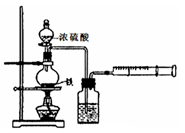
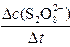 ��
��