��Ŀ����
����Ŀ����¯ú�����������ŷŵ�β��������H2��N2��CO��CO2��O2������N2ԼΪ55%��COԼΪ25%��CO2ԼΪ15%��O2ԼΪ1.64% (��Ϊ�������)��ij����С���β����Ӧ��չ���о���
��.ֱ����ȼ��
��֪��C(s)+O2(g)=CO2(g) ��H=-393.5kJ/mol�� 2C(s)+O2(g)=2CO(g) ��H=-221kJ/mol
(1)COȼ���ȵ��Ȼ�ѧ����ʽΪ__________________________________��
��. �����ϳɰ���ԭ��
��¯ú���������в����ת��Ϊ�ϳɰ���ԭ������
�����������н�������O2�����������з����ķ�Ӧ���£���������Ӧ��Ϊ���ȷ�Ӧ��
CO2+CH4![]() CO+H2 CO+H2O
CO+H2 CO+H2O![]() CO2+H2
CO2+H2
(2) ����ͨ����������¯��Ҫ�ϸ��¶ȣ��Ը��ݸ÷�Ӧ���������Ͳ��ýϸ��¶ȵ�ԭ
��________________________��
(3)ͨ��ͭ������¯������������V(H2)��V(N2)=______________________��
��.�ϳɰ��������Ӧ���о�
(4)�����������������ᣬ�ù����л����������Ⱦ��NOx��Ϊ���о���NOx�����������о�С���ں��������£���2L�����ܱ����������0.2molNO��0.1molCl2���������·�Ӧ��2NO(g)+Cl2(g) ![]() 2ClNO(g) ��H<0��10min ʱ��Ӧ��ƽ�⣬���10min��v (ClNO)=7.5��10-3mol/(L��min)����ƽ���n(Cl2)=___________mol��
2ClNO(g) ��H<0��10min ʱ��Ӧ��ƽ�⣬���10min��v (ClNO)=7.5��10-3mol/(L��min)����ƽ���n(Cl2)=___________mol��
���ʱNO��ת����Ϊ��1���������������䣬������Ӧ�ں�ѹ�����½��У�ƽ��ʱNO��ת����Ϊ��2����1_________��2 (�>������<����=��)��ƽ�ⳣ��K_______(�������С�����䡱��
(5)�������������Ʊ�NCl3��NCl3����ˮ�����֮һ����ǿ�����ԣ���ˮ������ܽ�ϡ�����е�NaClO2������ClO2���÷�Ӧ�����ӷ���ʽΪ__________________________ ��
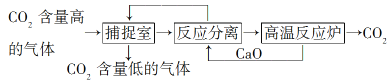
��.һ���ø�������(��Ҫ�ɷ�ΪSiO2��Al2O3��������Fe2O3)Ϊԭ���Ʊ���立�[NH4Al(SO4)2��12H2O]�Ĺ���������ͼ��ʾ���ش��������⣺
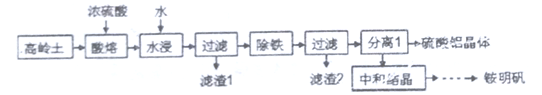
(6)�������ܡ�ʱ�䳬��40minʱ����Һ�е�Al2(SO4)3����SiO2��Ӧ����Al2O3��nSiO2�����������ܳ��ʽ��ͣ��÷�Ӧ�Ļ�ѧ����ʽΪ________________________��
(7)���顰���������������Ƿ�����ķ�����_________________________��
(8)���к͡�ʱ�����������Ϊ20���pH=2.8����ԭ����__________________________��
���𰸡� CO(g)+1/2O2(g)=CO2(g) ��H=-283kJ/mol �÷�Ӧ�������������ķ�Ӧ��ֻ���ڽϸ��¶��¦�G=��H-T��S ���п���С��0����Ӧ���������Է����� 88.36��55(��1.61��1��8��5) 0.025 < ���� HC1O+2C1O2-+H+=2ClO2��+Cl-+H2O Al2(SO4)3+nSiO2![]() Al2O3��nSiO2+3SO3�� ȡ������Һ���Թ���,�����е���KSCN��Һ,�۲���Һ�Ƿ��� ���������Al3+��ˮ��
Al2O3��nSiO2+3SO3�� ȡ������Һ���Թ���,�����е���KSCN��Һ,�۲���Һ�Ƿ��� ���������Al3+��ˮ��
��������(1)���C(s)+O2(g)=CO2(g) ��H=-393.5kJ/mol�� �� 2C(s)+O2(g)=2CO(g) ��H=-221kJ/mol ��
���ݸ�˹���ɿ�֪��-����1/2���õ�CO��ȼ���ȵ��Ȼ�ѧ����ʽ��CO(g)+1/2O2(g)=CO2(g) ��H=-283kJ/mol��
(2)���������з����ķ�ӦΪCO2+CH4=2CO+2H2��������H>0����S>0�����ݼ���˹��������G=��H-T��S��Ҫʹ��Ӧ���Է����У�������G<0����˱����ȡ�ϸߵ��¶ȣ��ʴ�Ϊ���÷�Ӧ�������������ķ�Ӧ��ֻ���ڽϸ��¶�����G=��H-T��S ���п���С��0����Ӧ���������Է����С�
(3)���¯ú����N2���Ϊ55����CO��CO2��H2������ֱ�Ϊ25��15��3.36����Ӧǰ��N2��������䣬����CO2+CH4=2CO+2H2��֪����CO��H2������ֱ�Ϊ30��30�������ܵ�CO�����Ϊ55������CO+H2O=CO2+H2�ɵ����ɵ�H2���Ϊ55�������ܵ�H2�����Ϊ3.36+30+55=88.36������ͨ��ͭ������¯������������V(H2)��V(N2)=88. 36��55=1.61��1��ԼΪ8��5���ʴ�Ϊ88.36��55(��1.61��1��8��5)��
(4) 10min�����ɵ�n(ClNO)=7.5��10-3��10��2mol=0.15mol �����Է�Ӧ��n(Cl2)=0.075mol ����ƽ���n(Cl2)=0.1mol-0.075mol=0.025mol���Է�Ӧ2NO(g)+Cl2(g) ![]() 2ClNO(g)�����ݵ������£���Ӧ��ϵ��ѹǿ�ڼ�С������Ϊ�ں�ѹ�����½��У����൱���ں��ݵĻ���������ѹǿ��ƽ�������ƶ���NO��ת����������1<��2���¶Ȳ��䣬ƽ�ⳣ��K���䡣
2ClNO(g)�����ݵ������£���Ӧ��ϵ��ѹǿ�ڼ�С������Ϊ�ں�ѹ�����½��У����൱���ں��ݵĻ���������ѹǿ��ƽ�������ƶ���NO��ת����������1<��2���¶Ȳ��䣬ƽ�ⳣ��K���䡣
(5) NCl3����ˮ�����֮һ����ǿ�����ԣ�����ˮ��Ĺ��ɣ�Cl�Ļ��ϼ۲�����Ϊ+1�ۣ�����ˮ�����ΪHClO��HClO��C1O2-����������ԭ��Ӧ����ClO2��ͬʱ����Cl-���ɣ����ӷ���ʽΪ��HC1O+2C1O2-+H+=2ClO2��+Cl-+H2O��
(6)��ʱ����ȣ���������Һ��Ϊ����������������ֽ�����Al2O3��SO3��Al2O3��SiO2�������Al2O3��nSiO2����ѧ����ʽΪ��Al2(SO4)3+nSiO2![]() Al2O3��nSiO2+3SO3����
Al2O3��nSiO2+3SO3����
(7)�����������ʹFe3+ת��ΪFe(OH)3������Ҫ���������������������Ƿ������ֻҪȡ�������������Һ�������е���KSCN��Һ���۲���Һ�Ƿ��죬��������ʾδ��������֮�Ѿ��������ʴ�Ϊ��ȡ������Һ���Թ����������е���KSCN��Һ,�۲���Һ�Ƿ��졣
(8)�кͽᾧʱ��Al3+�ᷢ��ˮ�⣬ˮ�������ȷ�Ӧ�����²�����ˮ��Ľ��У�Al3+ˮ������ԣ��ڽϵ͵�pH�£�H+Ũ�Ƚϴ�Ҳ��������Al3+��ˮ�⣬�ʴ�Ϊ�����������Al3+��ˮ�⡣