��Ŀ����
3��������Һ���й��������ʵ���Ũ�ȵıȽ���ȷ���ǣ�������| A�� | ������������Һ��0.1mol/L CH3COOH��Һ ��0.3mol/L CH3COOH��Һ��0.1mol/LNaOH��Һ������Ļ��Һ c��H+�����٣��� | |
| B�� | �����½�NaHCO3��NaHSO3�������ˮ��ǡ�ó����Ե���Һ�У�c��Na+��=c��HCO3-��+c��HSO3-��+2c��CO32-��+2c��SO32-�� | |
| C�� | ��ͬ�����£�pH=9�Ģ�CH3COONa��Һ����NH3•H2O��Һ����NaOH��Һ����ˮ�������c��OH-�����٣��ڣ��� | |
| D�� | ��֪����HF��CH3COOH�����ʵ���Ũ����ȵ�NaF��CH3COOK��Һ�У�[c��Na+��-c��F-��]��[c��K+��-c��CH3COO-��] |
���� A����������ΪCH3COOH��CH3COONa��������������ƴ�����룻
B���κε������Һ�ж����ڵ���غ㣬���ݵ���غ��жϣ�
C����������ˮ���룬���������ӵ��δٽ�ˮ���룬��������������Ũ����ͬʱ��������ˮ����̶���ͬ��
D���������Խǿ�����������ˮ��̶�ԽС���ٽ�������غ��жϣ�
��� A����������Ϊ0.1mol/L��CH3COOH��0.05mol/L��CH3COONa��������������ƴ�����룬����c��H+�����٣��ڣ���A����
B����Һ�����ԣ���c��H+��=c��OH-�����κε������Һ�ж����ڵ���غ㣬���ݵ���غ��c��Na+��=c��HCO3-��+c��HSO3-��+2c��CO32-��+2c��SO32-������B��ȷ��
C����������ˮ���룬���������ӵ��δٽ�ˮ���룬��������������Ũ����ͬʱ��������ˮ����̶���ͬ��������ˮ�������c��OH-�����٣���=�ۣ���C����
D���������Խǿ�����������ˮ��̶�ԽС������HF��CH3COOH�����������ˮ��̶�F-��CH3COO-������Һ��c��F-����c��CH3COO-�������������غ��c��Na+��=c��K+�������Ե�[c��Na+��-c��F-��]��[c��K+��-c��CH3COO-��]����D����
��ѡB��
���� ���⿼���˵������Һ������Ũ�ȴ�С�Ƚϣ�����غ������غ������������ʵ��������ˮ���ԭ��Ӧ�ã����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�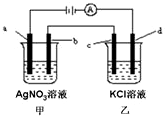 ��ͼ��ʾ��a��b��c��Ϊʯī�缫��dΪ̼�ֵ缫��ͨ����е�⣮�����ڵ������в���������ȫ���ݳ�������˵����ȷ���ǣ�������
��ͼ��ʾ��a��b��c��Ϊʯī�缫��dΪ̼�ֵ缫��ͨ����е�⣮�����ڵ������в���������ȫ���ݳ�������˵����ȷ���ǣ�������| A�� | ��b������5.4gʱ��d������������Ϊ2.24L | |
| B�� | ���ձ��е�d�缫��ӦʽΪFe-2e-=Fe2+ | |
| C�� | �����һ��ʱ����ס�������Һ��ϣ�һ����������� | |
| D�� | ���ձ�����Һ��pH���� |
| Ԫ�ش��� | A | B | D | E | G | I | J | K |
| ���ϼ� | -1 | -2 | +4��-4 | -1 | +5��-3 | +3 | +2 | +1 |
| ԭ�Ӱ뾶/nm | 0.071 | 0.074 | 0.077 | 0.099 | 0.110 | 0.143 | 0.160 | 0.186 |
| A�� | K������ˮ��Ӧ | |
| B�� | A��I��J�����Ӱ뾶�ɴ�С˳����A��J��I | |
| C�� | GԪ�صĵ��ʲ�����ͬ�������� | |
| D�� | J��DB2��ȼ���������ֻ����� |
| A�� | x-n | B�� | x+m | C�� | x-m | D�� | x+n |
| A�� | ����ˮ����ͭ�����Ҵ����Ƿ�ˮ | |
| B�� | ����ˮ�����������Ƿ��в�����֬���� | |
| C�� | ��Ũ��ˮϴ������������Ӧ���Թ� | |
| D�� | �ñ�����ˮ��Fe���¿��Ƶ��屽 |
 ������ԭ��Ӧ�����ӷ�Ӧ����ѧ��ѧ����Ҫ�ķ�Ӧ���ͣ��ش��������⣺
������ԭ��Ӧ�����ӷ�Ӧ����ѧ��ѧ����Ҫ�ķ�Ӧ���ͣ��ش��������⣺ =2Cr��OH��3��+3O2��+2H2O��
=2Cr��OH��3��+3O2��+2H2O��