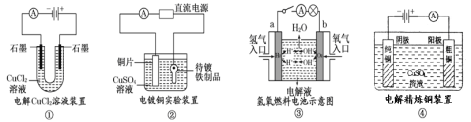
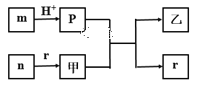
��Ŀ����
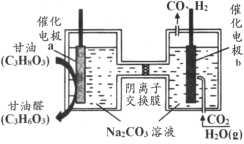
����Ŀ������ͼ��ʾװ�òⶨþ����Ʒ�е���þ���������������������ᷴӦ���������壩
���������գ�
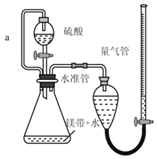
��1��������������Ŀ����_____
��2������a��������_____
��3��ȡ����þ����Ʒ�ֱ����ʵ�飬�������ݼ��±���
ʵ����� | þ��������g�� | ���������mL�����ѻ���ɱ�״���� |
1 | 0.053 | 44.60 |
2 | 0.056 | 47.05 |
����þ������������_____��������3λС����
��4������ⶨ���ƫ�ߣ����ܵ�ԭ����_____����ѡ���ţ�
a��װ��©��
b��δ��ȴ�����¼�����
c��þ���к�������þ
d��ĩ����ʱ�����ܵ�Һ�����ˮ��
���𰸡���֤þ����ȫ��Ӧ ƽ����ѹ��ʹ����˳�����£������������������������������������� 0.901 bd
��������
ʵ��ԭ��������þ�����������ʵ�����ȣ�ͨ���ų�Һ����������������������֪���������ʵ�����ͨ�����������ʵ������þ���������Ӷ������Ʒ��þ������������
��1��ʵ������Ҫͨ�����������ʵ������þ���������Ӷ������Ʒ��þ���������������Լ����������������ȷ��Mg��ȫ��Ӧ��
��2���淴Ӧ�Ľ��У���ƿ���������࣬ѹǿ����Һ©���е�����������£�Ϊ��ƽ����ѹ���õ���a����Һ©������ƿ�����������Ϳ���ʹ����˳�����¡����⣬���ڵ���������ռһ���������������a�����ⲿ����������ת�Ƶ���Һ©����Ӷ���������������������������������������Ե���a��������ƽ����ѹ��ʹ����˳�����º������������������������������������
��3��þ����ƽ������Ϊ��![]() ��0.0545g������������ƽ�����Ϊ��
��0.0545g������������ƽ�����Ϊ��![]() ��45.825mL�����ݷ�Ӧ��ϵʽMg��H2��֪��n��Mg����n��H2������Ʒ��Mg������Ϊ��24g/mol��
��45.825mL�����ݷ�Ӧ��ϵʽMg��H2��֪��n��Mg����n��H2������Ʒ��Mg������Ϊ��24g/mol��![]() ����0.04910������þ�����������ǣ�
����0.04910������þ�����������ǣ�![]() ��100%��0.901��
��100%��0.901��
��4��a��װ��©�����������������С���ⶨ���ƫ�ͣ���a����
b��δ��ȴ�����¼�������������������ԭ����֪���ⶨ���������ƫ�ⶨ���ƫ�ߣ���b��ȷ��
c��þ���к�������þ���������������ƫС���ⶨ���ƫ�ͣ���c����
d��ĩ����ʱ�����ܵ�Һ�����ˮ�ܣ����¶������ƫ�ⶨ���ƫ�ߣ���d��ȷ��

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�