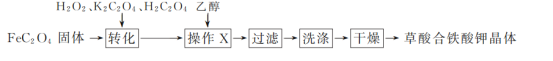
��Ŀ����
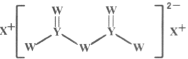
����Ŀ������������Ԫ��W��X��Y��Z��ԭ��������������X��ԭ�Ӱ뾶�����ж���������Ԫ�������ģ�W�ĺ����������X��Z������������֮����ȣ�Y��ԭ��������Z��������������2������W��X��Y����Ԫ���γɵĻ�����M�Ľṹ��ͼ��ʾ������������ȷ����
A.Ԫ�طǽ�����ǿ����˳��ΪW��Y��Z
B.Y���ʵ��۵����X����
C.������M��W��������8�����ȶ��ṹ
D.W�ļ��⻯���ȶ��Ա�Y�ļ��⻯���ȶ���ǿ
���𰸡�BD
��������
����������Ԫ��W��X��Y��Z��ԭ��������������X��ԭ�Ӱ뾶�����ж���������Ԫ�������ģ���XΪNaԪ�أ���W��X��Y����Ԫ���γɵĻ�̨��M�Ľṹ������֪��Y�γ��ĸ����ۼ�����YΪSiԪ�أ�Y��ԭ��������Z��������������2������Z��ClԪ�أ�W�ĺ����������X��Z������������֮����ȣ���WΪOԪ�ء�
A.ͬ����Ԫ�أ������ҷǽ�����������ǿ������Ԫ�صķǽ�����ǿ�ڹ�Ԫ�أ���A����
B.�赥��Ϊԭ�Ӿ��壬���кܸߵ��۵㣬������Ϊ�������壬�۵�ϵͣ���赥�ʵ��۵���ڽ����ƣ���B��ȷ��
C.������M�У�������ԭ�Ӻ�˫����ԭ�Ӷ�����8�����ȶ��ṹ����C����
D.Ԫ�صķǽ�����Խǿ�����⻯��Խ�ȶ�����Ԫ�صķǽ�����ǿ�ڹ�Ԫ�أ�����Ԫ�صļ��⻯���ȶ��Աȹ�Ԫ�صļ��⻯���ȶ���ǿ����D��ȷ��
��ѡBD��

����Ŀ������ͼ��ʾװ�òⶨþ����Ʒ�е���þ���������������������ᷴӦ���������壩
���������գ�
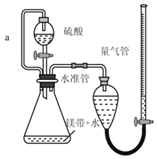
��1��������������Ŀ����_____
��2������a��������_____
��3��ȡ����þ����Ʒ�ֱ����ʵ�飬�������ݼ��±���
ʵ����� | þ��������g�� | ���������mL�����ѻ���ɱ�״���� |
1 | 0.053 | 44.60 |
2 | 0.056 | 47.05 |
����þ������������_____��������3λС����
��4������ⶨ���ƫ�ߣ����ܵ�ԭ����_____����ѡ���ţ�
a��װ��©��
b��δ��ȴ�����¼�����
c��þ���к�������þ
d��ĩ����ʱ�����ܵ�Һ�����ˮ��