��Ŀ����
����Ŀ�����������(Na2S2O3)���������մ�����������ɫ���ĵ�б���壬�۵�48�档���������(Na2S2O3)����Ϊ����ҵ�Ķ�Ӱ������Ӧԭ��ΪAgBr+2Na2S2O3=Na3[Ag(S2O3)2]+NaBr��
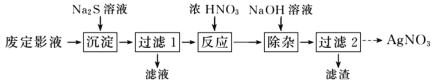
��Ϊ�˴ӷ϶�ӰҺ����ȡ AgNO3���������ʵ�����̡�
(1)������������������ Ag2S ���������������ȫ�IJ�����________��
(2)����Ӧ�������л����ɵ���ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ________��
(3)������ 2������Һ��ȡ AgNO3����IJ���������Ũ������ȴ�ᾧ��________��________�����
����ͼ��ʵ����ģ�ҵ�Ʊ� Na2S2O3 ��װ��ͼ��
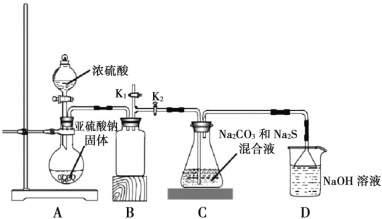
����ͼʾ�ش��������⣺
(4)װ�� A ��ʢ���������ƹ���IJ�������������________��װ�� B ��������________��
(5)��Һ©������ֱ���� 98����Ũ���ᣬ��ƿ�й����ײ�������顱����ʹ��Ӧ���ʱ�������������顱�����ԭ����________��
(6)���� K1���ܵ�Ŀ����Ϊ�˷�ֹ���װ��ʱ��ɿ�����Ⱦ���������������________��
(7)��������ƻ������ڳ�ȥ����Ƥ��ʱ�������ظ����Σ����仹ԭ�� Cr3+�������ϴ���1mol Cr2O72-��Ҫ Na2S2O3������Ϊ________��
���𰸡����ã�ȡ�ϲ���Һ�μ�Na2S��Һ���������г������ɣ���˵�������Ѿ���ȫ Ag2S+4HNO3=2AgNO3+2NO2��+S+2H2O ���� ϴ�� Բ����ƿ ��ֹ����(��ȫƿ) ���ɵ�Na2SO4���帽����Na2SO3���� ���齺�ܽ�K1�϶�����ͨ��װ��NaOH��Һ���ձ��У�Ȼ��ر�K2��K1 118.5g
��������
�ӷ϶�ӰҺ����ȡAgNO3���ڷ϶�ӰҺ{��Ҫ�ɷ�ΪNa3[Ag(S2O3)2]}�м���Na2S��Һ����Ag2S���������˺���Ũ�����ܽ�Ag2S����������������Һ�͵���ɫ���壬�õ���ɫ����Ϊ��������NaOH��ȥ���ʣ����˺�õ���������Һ��Ȼ����������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ����������壻
��Aװ����Ũ�������������ƹ��巴Ӧ�ų������������壬���ڶ�������������ˮ��Bװ��Ϊ��ȫƿ��Cװ���ж�����������ͨ��̼���ƺ����ƵĻ����Һ�з�Ӧ����Na2S2O3��Һ��δ��Ӧ��ȫ�Ķ���������D�б�����������Һ���գ���ֹ��Ⱦ���ݴ˷������
��(1)������������������Ag2S���������������ȫ�IJ���Ϊ�����ã�ȡ�ϲ���Һ�μ�Na2S��Һ���������г������ɣ���˵�������Ѿ���ȫ���ʴ�Ϊ�����ã�ȡ�ϲ���Һ�μ�Na2S��Һ���������г������ɣ���˵�������Ѿ���ȫ��
(2) ��Ũ�����ܽ�Ag2S����������������Һ����ͬʱ�ų������������壬��Ӧ�Ļ�ѧ����ʽΪAg2S+4HNO3=2AgNO3+2NO2��+S+2H2O���ʴ�Ϊ��Ag2S+4HNO3=2AgNO3+2NO2��+S+2H2O��
(3)������ 2������Һ��ȡAgNO3����IJ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ʴ�Ϊ�����ˣ�ϴ�ӣ�
��(4)����ͼʾ��װ�� A ��ʢ���������ƹ���IJ�������ΪԲ����ƿ����������������ˮ��װ�� B Ϊ��ȫƿ����ֹ�������ʴ�Ϊ��Բ����ƿ����ֹ����(��ȫƿ)��
(5)��Һ©������ֱ���� 98����Ũ���ᣬ��ƿ�й����ײ�������顱�������ɵ�Na2SO4���帽����Na2SO3���棬ʹ��Ӧ���ʱ������ʴ�Ϊ�����ɵ�Na2SO4���帽����Na2SO3���棻
(6)����K1���ܵ�Ŀ����Ϊ�˷�ֹ���װ��ʱ��ɿ�����Ⱦ�������������Ϊ�����齺�ܽ�K1�϶�����ͨ��װ��NaOH��Һ���ձ��У�Ȼ��ر�K2��K1���ʴ�Ϊ�����齺�ܽ�K1�϶�����ͨ��װ��NaOH��Һ���ձ��У�Ȼ��ر�K2��K1��
(7)��������ƻ������ڳ�ȥ����Ƥ��ʱ�������ظ����Σ����仹ԭ��Cr3+����Ӧ�ķ���ʽΪ3S2O32-+ 4Cr2O72-+ 26H+= 6SO42-+8Cr3++ 13H2O�����ݷ���ʽ�������ϴ���1mol Cr2O72-��Ҫ0.75mol Na2S2O3������Ϊ0.75mol��158g/mol=118.5g���ʴ�Ϊ��118.5g��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ͼ��ʾװ�òⶨþ����Ʒ�е���þ���������������������ᷴӦ���������壩
���������գ�
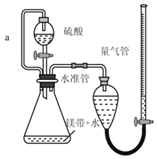
��1��������������Ŀ����_____
��2������a��������_____
��3��ȡ����þ����Ʒ�ֱ����ʵ�飬�������ݼ��±���
ʵ����� | þ��������g�� | ���������mL�����ѻ���ɱ�״���� |
1 | 0.053 | 44.60 |
2 | 0.056 | 47.05 |
����þ������������_____��������3λС����
��4������ⶨ���ƫ�ߣ����ܵ�ԭ����_____����ѡ���ţ�
a��װ��©��
b��δ��ȴ�����¼�����
c��þ���к�������þ
d��ĩ����ʱ�����ܵ�Һ�����ˮ��