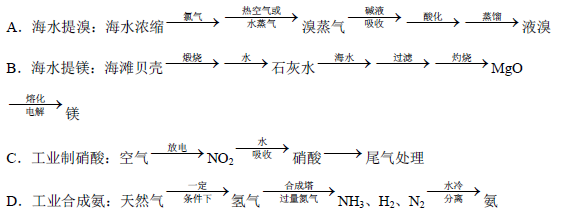
��Ŀ����
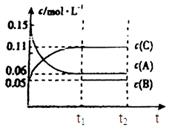
һ���¶���,��2 L���ܱ�������,X��Y��Z�������������ʱ��仯��������ͼ��ʾ: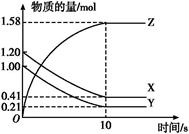
(1)�ӷ�Ӧ��ʼ��10 sʱ,��Z��ʾ�ķ�Ӧ����Ϊ����������������,X�����ʵ���Ũ�ȼ�������������,Y��ת����Ϊ������
(2)�÷�Ӧ�Ļ�ѧ����ʽΪ��
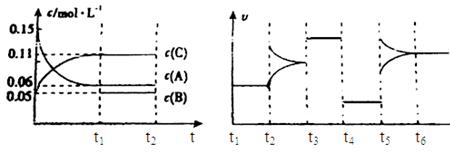
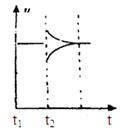
(3)10 s���ijһʱ��(t1)�ı����������,��������ʱ��ı仯ͼ����ͼ��ʾ: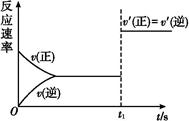
������˵�����ϸ�ͼ���������������
| A��t1ʱ��,������X��Ũ�� | B��t1ʱ��,��������ϵ�¶� |
| C��t1ʱ��,��С��������� | D��t1ʱ��,ʹ���˴��� |
(1)0.079 mol��L-1��s-1��0.395 mol��L-1��79.0%
(2)X(g)+Y(g) 2Z(g)
2Z(g)
(3)CD
����

��ϰ��ϵ�д�
�����Ŀ
��ӦA��3B=2C��2D�����ֲ�ͬ����µķ�Ӧ���ʿɷֱ�Ϊ
��v
| A����0.15 mol/(L��s)����v | B����0.6 mol/(L��s)����v | C����0.4 mol/(L��s)����v | D����0.45 mol/(L��s) |
 2B(g)����H��-a kJ��mol��1������B�����ʵ���Ũ����ʱ��仯��ͼ��ʾ��
2B(g)����H��-a kJ��mol��1������B�����ʵ���Ũ����ʱ��仯��ͼ��ʾ��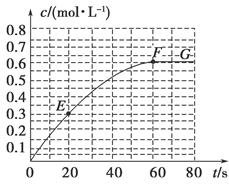
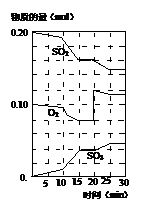
 TaI4��g����S2��g������H>0��I��
TaI4��g����S2��g������H>0��I��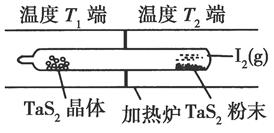
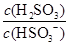 ��________���������С�����䡱����
��________���������С�����䡱���� 2NH3 ��H<0���������¶Ȳ��䣬ij��ȤС��ͬѧ��÷�Ӧ������������ѹǿ��ʱ��仯��ͼ��ʾ��8 min�ڷ���NH3��ƽ����������Ϊ mol��L-1��min-1��
2NH3 ��H<0���������¶Ȳ��䣬ij��ȤС��ͬѧ��÷�Ӧ������������ѹǿ��ʱ��仯��ͼ��ʾ��8 min�ڷ���NH3��ƽ����������Ϊ mol��L-1��min-1��
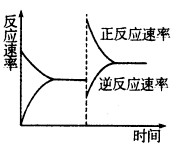
 H=-akJ��mol-1
H=-akJ��mol-1 C(g)��D(g) ��H��0����ش���������:
C(g)��D(g) ��H��0����ش���������: