��Ŀ����
6����ϩ��ʯ���ѽ�������Ҫ�ɷ֣����IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����ش��������⣮��1����ϩ�ĵ���ʽ
 ���ṹ��ʽH2C=CH2��
���ṹ��ʽH2C=CH2����2�����������У�����ͨ����ϩ�ӳɷ�Ӧ�õ�����B������ţ���
A��CH3CH3��������������B��CH3CHCl2 C��CH3CH2OH��������D��CH3CH2Br
��3������������ϩ���Լ���BD������ţ���
A��ϡ���ᡡ��������B��������Ȼ�̼��Һ��������C��ˮ��������D�����Ը��������Һ
��4����֪��2CH3CHO+O2$��_{��}^{����}$2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·����ͼ��ʾ��

д��A������Ʒ�Ӧ�ķ���ʽ2CH3CH2OH+2Na��2CH3CH2ONa+H2������Ӧ�ڵĻ�ѧ����ʽΪ2CH3CH2OH+O2$��_{��}^{Cu��Ag}$2CH3CHO+2H2O����ҵ������ϩΪԭ�Ͽ�������һ����Ҫ�ĺϳ��л��߷��ӻ�����䷴Ӧ�Ļ�ѧ����ʽΪ
 ����Ӧ�����ǼӾ۷�Ӧ��
����Ӧ�����ǼӾ۷�Ӧ��
���� ��1����ϩ�Ǻ���̼̼˫�������ϩ�������ݵ���ʽ������д�ṹ��ʽ��
��2����ϩ�ӳɷ�Ӧ��������ԭ�ӻ�ԭ���ż���˫��̼ԭ���ϣ�˫����Ϊ������
��3����ϩ�к���̼̼˫�������Է����ӳɷ�Ӧ�����Ա�ǿ�����������������鲻�ܣ�
��4����ϩ���Ժ�ˮ�ӳ������Ҵ����Ҵ��������Ʒ�Ӧ�����������Ҵ�Ҳ���Ա�����Ϊ��ȩ����ȩ�ױ�����Ϊ�����ϩ���Է����Ӿ۷�Ӧ���ɾ���ϩ��
��� �⣺��1����ϩ�к�̼��̼֮���Թ���˫����ϣ�����ʽΪ�� �����ݵ���ʽ������д�ṹ��ʽΪH2C=CH2��
�����ݵ���ʽ������д�ṹ��ʽΪH2C=CH2��
�ʴ�Ϊ�� ��H2C=CH2��
��H2C=CH2��
��2����ϩ���廯�ⷢ���ӳ����������飬��ˮ�ӳ������Ҵ����������ӳ��������飬
�ʴ�Ϊ��B��
��3����ϩ�к���̼̼˫�������Է����ӳɷ�Ӧ��ʹ��ˮ��ɫ�����Ա�ǿ��������������������Ӷ�ʹ���������ɫ�������鲻�ܣ�
�ʴ�Ϊ��BD��
��4����ϩ���Ժ�ˮ�ӳ������Ҵ�������A���Ҵ����Ҵ����Ա�����ΪB��ȩ����ȩ�ױ�����ΪC���ᣬ�Ҵ��������Ʒ�Ӧ������������Ӧ����ʽΪ2CH3CH2OH+2Na��2CH3CH2ONa+H2�����Ҵ��Ĵ�������ӦΪ��2CH3CH2OH+O2$��_{��}^{Cu��Ag}$2CH3CHO+2H2O����ϩ���Է����Ӿ۷�Ӧ���ɾ���ϩ����ӦΪ�� �����ڼӾ۷�Ӧ��
�����ڼӾ۷�Ӧ��
�ʴ�Ϊ��2CH3CH2OH+2Na��2CH3CH2ONa+H2����2CH3CH2OH+O2$��_{��}^{Cu��Ag}$2CH3CHO+2H2O�� ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ��
���� ������Ҫ����ѧ����ϩ�Ļ�ѧ���ʣ����Ը��ݽ̲�֪ʶ���ش��ѶȲ���

| A�� | ȷ��ȡ24.00 mL��Һ���ѡ����Һ�ܡ���Ͳ��ζ��� | |
| B�� | �ù㷺pH��ֽ���ij��ˮ��pHΪ2 | |
| C�� | ͨ�����ò������ݵĿ��������Ƚϲ�ͬ������Na2S2O3��Һ��ϡ����ķ�Ӧ���� | |
| D�� | ���ʵķ����ᴿ����֮һΪ��ɸ�֡����罺��-�����������ᴿ������Һ-���˷��� |
| A�� | �����¶ȣ�ƽ�������ƶ� | B�� | m��p������ӦΪ���ȷ�Ӧ | ||
| C�� | m+n��p������ӦΪ���ȷ�Ӧ | D�� | ��Сѹǿ��ƽ�������ƶ� |
| A�� | ����ˮ��Ӧ��������ˮ��Ӧ���� | B�� | �����Դ�KCl��Һ���û����� | ||
| C�� | Ba��OH��2����NH4Cl��Ӧ����Ӧ���� | D�� | ���ԣ�KOH��Ba��OH��2��NaOH |

| A�� | Fe�缫������������������Ӧ | |
| B�� | Cl-��ʯī���������˶� | |
| C�� | ʯī�缫��Ӧ��Fe3++3e-�TFe | |
| D�� | ���ط����ܷ�Ӧ��2Cl${\;}_{\;}^{-}$+2Fe3+�TCl2+2Fe2+ |
| A�� | NaͶ��ˮ�У�Na+H2O�TNa++OH-+H2�� | |
| B�� | NaHCO3��Һ������������Һ��Ӧ��HCO3-+2OH-�TCO2��+H2O | |
| C�� | ��CH3COOH ��ҺCaCO3��CaCO3+2H+�TCa2++H2O+CO2�� | |
| D�� | ��NaAlO2��Һ��ͨ�����CO2��Al��OH��3��CO2+AlO2-+2H20�TAl��OH��3��+HCO3- |
| A�� | H2O2�ĵ���ʽ�� | B�� | CS2�ı���ģ�ͣ� | ||
| C�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | D�� | ���ķ���ʽ��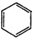 |
| A�� | ����1molFe��OH��3��������������������NA��Fe��OH��3���� | |
| B�� | 1mol-L-1Na2CO3��Һ100mL������0.1NA��CO32- | |
| C�� | ����������1molCl2��ַ�ӳת�Ƶĵ�����Ϊ3NA | |
| D�� | ���³�ѹ�£�22.4L��һ�ȼ����к��еķ�����С��NA�� |
| A�� | ����0.1molFeCl3����Һ�μӵ���ˮ����ȫˮ�������0.1NA��Fe��OH��3���� | |
| B�� | ���³�ѹ�£�0.1molD216O�к�������������������������ΪNA | |
| C�� | ��0.1molCl2ͨ��1Lˮ�У�ת�Ƶĵ�����Ϊ0.1NA | |
| D�� | ��ⱥ��ʳ��ˮ������������1.12LH2ʱ��ת�Ƶĵ�����һ��Ϊ0.1NA |