��Ŀ����
����Ŀ��FeSe ��MgB2�ȳ������Ͼ��й�����Ӧ��ǰ����
��1����̬Feԭ�Ӽ۲���ӵĵ����Ų�ͼ���������ʽ��Ϊ________����̬Seԭ�ӵĵ���ռ������ܼ��ĵ���������ͼΪ________�Ρ�
��2����FeSe��Ƕ����ण�![]() ���ܵõ������������ܵij������ϡ�����е�ԭ�ӵ��ӻ�����Ϊ________���÷����ڴ���________�����ţ���
���ܵõ������������ܵij������ϡ�����е�ԭ�ӵ��ӻ�����Ϊ________���÷����ڴ���________�����ţ���
A���Ҽ� B���м� C����λ�� D�����
��3���������ֱ������Һ�����õ����кܸ߷�Ӧ���ԵĽ���������Һ����ͨ��ϵ�з�Ӧ���Ƶ�FeSe����������Li0.6(NH2)0.2(NH3)0.8Fe2Se2��
��NH2���Ŀռ乹��Ϊ________��
��Һ���ǰ���Һ���IJ���,������Һ����ԭ����________��
�۽��������Һ��ʱ������Ӧ��Li + (m+n)NH3=X+e��(NH3)n��X�Ļ�ѧʽΪ________��
��4��MgB2����ṹ��ͼ��ʾ��Bԭ�Ӷ���Ϊһ�㣬����������ʯī�Ľṹ��ÿ��Bԭ����Χ����________����֮�Ⱦ����������Bԭ�ӣ��������ױ߱߳�Ϊa cm����Ϊc cm�������ӵ�������ֵΪNA ���þ�����ܶ�Ϊ________ g��cm��3���г�����ʽ����

���𰸡� ���壨�Ĵ��� sp2 �ӻ� AB v �� �����Ӽ������������Ӽ���������ǿ������Һ�� Li(NH3)m+ 3
���壨�Ĵ��� sp2 �ӻ� AB v �� �����Ӽ������������Ӽ���������ǿ������Һ�� Li(NH3)m+ 3 ![]()
��������
��1��Feԭ�Ӻ˵����Ϊ26����̬Feԭ�ӵ����Ų�ʽΪ1s22s22p63s23p63d64s2��Seԭ�Ӻ˵����Ϊ34����̬Seԭ�ӵ����Ų�ʽΪ1s22s22p63s23p63d104s24p4���Դ˷������
��2�����N������̼�ɼ�����һ��δ�ɶԵ��Ӻ��������̼ԭ���γɴ�м�����˷��������Ե�Sp2�ӻ����÷����ڴ��ڦҼ��ͦм���
��3����NH2����Nԭ�Ӽ۲���Ӷ���=![]() =4����2�Թµ��Ӷԣ����Կռ乹��Ϊv �ͣ�
=4����2�Թµ��Ӷԣ����Կռ乹��Ϊv �ͣ�
�ڰ����Ӽ������������Ӽ���������ǿ������Һ����
��Li + (m+n)NH3=X+e��(NH3)n������ԭ���غ�͵���غ��ȷ��X�Ļ�ѧʽ��
��4������Bԭ�Ӷ���Ϊһ�㣬����������ʯī�Ľṹ����ʯī������ÿ��̼ԭ��������3��̼ԭ�ӵȾ���������ʸþ�����ÿ��Bԭ����Χ����3����֮�Ⱦ����������Bԭ�ӣ�����![]() =
=![]() ���㾧����ܶȡ�
���㾧����ܶȡ�
��1��Feԭ�Ӻ˵����Ϊ26����̬Feԭ�ӵ����Ų�ʽΪ1s22s22p63s23p63d64s2�����̬Feԭ�ӵĺ���۵����Ų�ͼΪ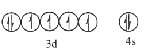 ��Seԭ�Ӻ˵����Ϊ34����̬Seԭ�ӵ����Ų�ʽΪ1s22s22p63s23p63d104s24p4����̬Seԭ�ӵ���ռ�ݵ��ܼ���1s��2s��2p��3p��3d��4s��4P������ܼ�Ϊ4p�������������ͼΪ�����Σ�
��Seԭ�Ӻ˵����Ϊ34����̬Seԭ�ӵ����Ų�ʽΪ1s22s22p63s23p63d104s24p4����̬Seԭ�ӵ���ռ�ݵ��ܼ���1s��2s��2p��3p��3d��4s��4P������ܼ�Ϊ4p�������������ͼΪ�����Σ�
�ʴ�Ϊ�� �����壻
�����壻
��2�����N������̼�ɼ�����һ��δ�ɶԵ��Ӻ��������̼ԭ���γɴ�м�����˷��������Ե�Sp2�ӻ����÷����ڴ��ڦҼ��ͦм�����������λ�����������ѡAB��
�ʴ�Ϊ��sp2 �ӻ���AB��
��3����NH2����Nԭ�Ӽ۲���Ӷ���=![]() =4����2�Թµ��Ӷԣ����Կռ乹��Ϊv �ͣ�
=4����2�Թµ��Ӷԣ����Կռ乹��Ϊv �ͣ�
�ڰ����Ӽ������������Ӽ���������ǿ������Һ����
��Li + (m+n)NH3=X+e��(NH3)n������ԭ���غ�͵���غ��ȷ��X�Ļ�ѧʽΪLi(NH3)m+��
�ʴ�Ϊ��v �ͣ������Ӽ������������Ӽ���������ǿ������Һ����Li(NH3)m+��
��4��������Mg�ĸ���Ϊ12![]() +2
+2![]() =3��B�ĸ���Ϊ6���������=6
=3��B�ĸ���Ϊ6���������=6![]() cm3=6
cm3=6![]() cm3�������ܶ�=
cm3�������ܶ�=![]() =
=![]() g��cm��3��
g��cm��3��
�ʴ�Ϊ��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����������о���Ա������ú��������Ȼ�ѧѭ��ʵ��̫���ܵ�ת����洢���������£�
��1����Ӧ��2H2SO4(l)=2SO2(g)+2H2O(g)+O2(g) ��H1=+551 kJ��mol��1
��Ӧ��S(s)+O2(g)=SO2(g) ��H3=��297 kJ��mol��1
��Ӧ�����Ȼ�ѧ����ʽ��________________________________��
��2��I��������Ϊˮ��Һ��SO2�绯��Ӧ�Ĵ��������ܵĴ��������¡���ii����������
i��SO2+4I��+4H+=S��+2I2+2H2O
ii��I2+2H2O+_________=_________+_______+2 I��_____________
��3��̽��i��ii��Ӧ������SO2�绯��Ӧ���ʵĹ�ϵ��ʵ�����£��ֱ�18 mL SO2������Һ���뵽2 mL�����Լ��У��ܱշ��ù۲�������֪��I2���ܽ���KI��Һ�У�
��� | A | B | C | D |
�Լ���� | 0.4 mol��L��1 KI | a mol��L��1 KI 0.2 mol��L��1 H2SO4 | 0.2 mol��L��1 H2SO4 | 0.2 mol��L��1 KI 0.0002 mol I2 |
ʵ������ | ��Һ��ƣ�һ��ʱ�����ֻ��� | ��Һ��ƣ����ֻ��ǽ�A�� | ���������� | ��Һ���غ�ɫ�ܿ���ɫ����ɻ�ɫ�����ֻ��ǽ�A�� |
��B��A�ĶԱ�ʵ�飬��a=__________��
�ڱȽ�A��B��C���ɵó��Ľ�����______________________��
��ʵ�������SO2���绯��Ӧ����D��A�����i��ii��Ӧ���ʽ���ԭ��________________��



