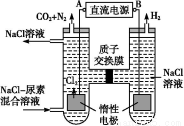
��Ŀ����
Ϊ��СCO2�Ի�����Ӱ��,�ڳ�������̼����ͬʱ,�����ǿ��CO2�������õ��о���T1 ��ʱ,��9 mol CO2 ��12 mol H2����3 L�ܱ�������,������ӦCO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H��0,������CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ,ƽ��ʱ������ѹǿΪp1���ı�ijһ�������½���������Ӧ,CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ������˵��������ǣ� ��
CH3OH(g)+H2O(g) ��H��0,������CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ,ƽ��ʱ������ѹǿΪp1���ı�ijһ�������½���������Ӧ,CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ������˵��������ǣ� ��

A.���ߢ��Ӧ�������ı�������ѹǿ
B.T2��ʱ,������Ӧƽ�ⳣ��Ϊ0.42,��T2��T1
C.��T1��,����ʼʱ�������г���5 mol CO2��5 mol H2��5 mol CH3OH(g)��5 mol H2O(g),���ƽ��ǰv(��)��v(��)
D.��T1��,����ʼʱ����������4.5 mol CO2��6 mol H2,ƽ��ʱ������ѹǿp=
D
��������������ѹǿ,���ʼӿ�,ƽ������,��CH3OH�����ʵ�������,A��;��ͼ֪,T1���´�ƽ��ʱCH3OHΪ3 mol,��������CO2Ϊ6 mol��H2Ϊ3 mol��H2OΪ3 mol,�����ʵ�ƽ��Ũ��:CO2Ϊ2 mol/L��H2Ϊ1 mol/L��H2OΪ1 mol/L��CH3OHΪ1 mol/L,���ƽ�ⳣ��Ϊ0.5,��÷�Ӧ�������,�����������ƶ�,ƽ�ⳣ����С,����,��T2��ʱƽ�ⳣ��Ϊ0.42,��T2��T1,B��;��T1��,����ʼʱ�������г���5 mol CO2��5 mol H2��5 mol CH3OH(g)��5 mol H2O(g),Q=0.36��0.5,���������,��ƽ��ǰv(��)��v(��),C��;��T1��,����Ͷ�Ϸ�ʽ������Ӧ���ת�������,��p=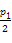 ,�������ڸ÷�Ӧ�������������С�ķ�Ӧ,����ʼ���뷴Ӧ������ʵ����ٵ�,��ת����С,��p��
,�������ڸ÷�Ӧ�������������С�ķ�Ӧ,����ʼ���뷴Ӧ������ʵ����ٵ�,��ת����С,��p��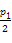 ,D����
,D����

 �����ߴ���ϵ�д�
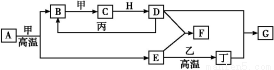
�����ߴ���ϵ�д�T �桢2 L�ܱ�������ijһ��Ӧ�ڲ�ͬʱ�̵ĸ����ʵ�����ͼ��ʾ(EΪ����,����Ϊ����)���ش��������⡣

(1)д���÷�Ӧ�Ļ�ѧ����ʽ: ��
(2)��Ӧ��ʼ��3 minʱ,��D��ʾ��ƽ����Ӧ����Ϊ mol��L-1��min-1��
(3)T ��ʱ,�÷�Ӧ�Ļ�ѧƽ�ⳣ��K= ��
(4)��6 minʱ,�����¶Ȳ���,�������������С��ԭ����һ��,���´ﵽƽ���,D���������Ϊ ��
(5)����һ��2 L���ܱ�����,T �桢ijһʱ��,�����и����ʵ����ʵ��������ʾ��
���� | A | B | D | E |
���ʵ���(mol) | 0.8 | 1.0 | 0.4 | 0.2 |
��ʱv(��) v(��)(����ڡ��������ڡ���С�ڡ�)��