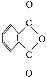
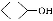
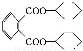
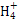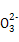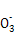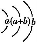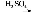ΧβΡΩΡΎ»ί
T ΓφΓΔ2 LΟή±’»ίΤς÷–Ρ≥“ΜΖ¥”Π‘Ύ≤ΜΆ§ ±ΩΧΒΡΗςΈο÷ ΒΡΝΩ»γΆΦΥυ Ψ(EΈΣΙΧΧε,Τδ”ύΈΣΤχΧε)ΓΘΜΊ¥πœ¬Ν–Έ ΧβΓΘ
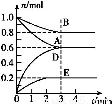
(1)–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ: ΓΘ
(2)Ζ¥”ΠΩΣ Φ÷Ν3 min ±,”ΟD±μ ΨΒΡΤΫΨυΖ¥”ΠΥΌ¬ ΈΣ molΓΛL-1ΓΛmin-1ΓΘ
(3)T Γφ ±,ΗΟΖ¥”ΠΒΡΜ·―ßΤΫΚβ≥Θ ΐK= ΓΘ
(4)ΒΎ6 min ±,±Θ≥÷Έ¬Ε»≤Μ±δ,ΫΪ»ίΤςΒΡΧεΜΐΥθ–Γ÷Ν‘≠ά¥ΒΡ“ΜΑκ,÷Ί–¬¥οΒΫΤΫΚβΚσ,DΒΡΧεΜΐΖ÷ ΐΈΣ ΓΘ
(5)Νμ”–“ΜΗω2 LΒΡΟή±’»ίΤς,T ΓφΓΔΡ≥“Μ ±ΩΧ,»ίΤς÷–ΗςΈο÷ ΒΡΈο÷ ΒΡΝΩ»γ±μΥυ ΨΓΘ
Έο÷ | A | B | D | E |
Έο÷ ΒΡΝΩ(mol) | 0.8 | 1.0 | 0.4 | 0.2 |
¥Υ ±v(’ΐ) v(Ρφ)(ΧνΓΑ¥σ”ΎΓ±ΓΔΓΑΒ»”ΎΓ±ΜρΓΑ–Γ”ΎΓ±)ΓΘ
(1)2A(g)+B(g) 3D(g)+E(s)
3D(g)+E(s)
(2)0.1 (3)0.75 (4)30% (5)¥σ”Ύ
ΓΨΫβΈωΓΩ(1)3 min ±,AΓΔBΓΔDΓΔEΒΡΈο÷ ΒΡΝΩ±δΜ·ΝΩΖ÷±πΈΣ0.4 molΓΔ0.2 molΓΔ0.6 molΓΔ0.2 mol,Ι ΤδΜ·―ßΦΤΝΩ ΐ÷°±»ΈΣ2ΓΟ1ΓΟ3ΓΟ1,“ρ¥ΥΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ2A(g)+B(g) 3D(g)+E(s)ΓΘ
3D(g)+E(s)ΓΘ
(2)v(D)=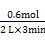 =0.1 molΓΛL-1ΓΛmin-1ΓΘ
=0.1 molΓΛL-1ΓΛmin-1ΓΘ
(3)3 min ±,AΓΔBΓΔDΒΡΈο÷ ΒΡΝΩΖ÷±πΈΣ0.6 molΓΔ0.8 molΓΔ0.6 mol,‘ρK=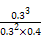 =0.75ΓΘ
=0.75ΓΘ
(4)“ρΖ¥”Π«ΑΚσΤχΧεΧεΜΐ≤Μ±δ,Υυ“‘Υθ–ΓΧεΜΐ(‘ω¥σ―Ι«Ω)ΤΫΚβ≤Μ“ΤΕ·,DΒΡΧεΜΐΖ÷ ΐ”κ3 min ±œύΒ»,ΈΣ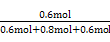 ΓΝ100%=30%ΓΘ
ΓΝ100%=30%ΓΘ
(5)Ω…ΦΤΥψ≥ωQ=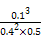 =0.0125ΘΦ0.75,Ι ΤΫΚβ’ΐœρ“ΤΕ·,v(’ΐ)¥σ”Ύv(Ρφ)ΓΘ
=0.0125ΘΦ0.75,Ι ΤΫΚβ’ΐœρ“ΤΕ·,v(’ΐ)¥σ”Ύv(Ρφ)ΓΘ

 Οϊ ΠΒψ≤ΠΨμœΒΝ–¥πΑΗ
Οϊ ΠΒψ≤ΠΨμœΒΝ–¥πΑΗ ”Δ≤≈ΦΤΜ°ΤΎΡ©Βς―–œΒΝ–¥πΑΗ
”Δ≤≈ΦΤΜ°ΤΎΡ©Βς―–œΒΝ–¥πΑΗ”–ΙΊ‘ΣΥΊXΓΔYΓΔZΓΔWΒΡ–≈œΔ»γœ¬:
‘ΣΥΊ | ”–ΙΊ–≈œΔ |
X | ‘≠Ή”ΑκΨΕΈΣ0.074 nm,Τδ«βΜ·ΈοΒΡΫαΙΙΡΘ–ΆΩ…±μ ΨΈΣ: |
Y | ‘≠Ή”ΑκΨΕΈΣ0.102 nm,Τδ‘≠Ή”ΚΥΆβΒγΉ”≈≈≤ΦΈΣ: |
Z | »ΥΧε÷–±Ί–ηΈΔΝΩ‘ΣΥΊ÷–Κ§ΝΩΉνΕύ,ΧεΡΎ»± ßΜα“ΐΤπΤΕ―Σ |
W | Υυ‘Ύ÷ςΉε–ρ ΐ”κΥυ‘Ύ÷ήΤΎ–ρ ΐ÷°≤νΈΣ4 |
«κΜΊ¥πœ¬Ν–Έ Χβ:
(1)W‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο « ΓΘ
(2)Y”κWœύ±»,Ζ«Ϋπ τ–‘Ϋœ«ΩΒΡ « (Χν‘ΣΥΊΖϊΚ≈),œ¬Ν– ¬ ΒΡή÷ΛΟς’β“ΜΫα¬έΒΡ « (ΧνΉ÷ΡΗ)ΓΘ
a.W‘ΣΥΊ«βΜ·ΈοΒΡΈ»Ε®–‘¥σ”ΎY‘ΣΥΊ«βΜ·ΈοΒΡΈ»Ε®–‘
b.W‘ΣΥΊ«βΜ·ΈοΥ°»ή“ΚΒΡΥα–‘«Ω”ΎY‘ΣΥΊ«βΜ·ΈοΥ°»ή“ΚΒΡΥα–‘
c.W‘ΣΥΊΒΡΒΞ÷ Ρή”κY‘ΣΥΊΒΡ«βΜ·ΈοΖ¥”Π,÷ΟΜΜ≥ωYΒΞ÷
d.WΒΡΉνΗΏΦέΚ§―θΥα±»YΒΡΉνΗΏΦέΚ§―θΥαΒΡΥα–‘«Ω
(3)Z‘ΣΥΊΚΆX‘ΣΥΊΩ…–Έ≥…άκΉ”Z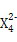 ,Κ§ΗΟάκΉ”ΒΡ―Έ «”≈ΝΦΒΡ¬Χ…ΪœϊΕΨΦΝΚΆΈόΜζ–θΡΐΦΝΓΘ
,Κ§ΗΟάκΉ”ΒΡ―Έ «”≈ΝΦΒΡ¬Χ…ΪœϊΕΨΦΝΚΆΈόΜζ–θΡΐΦΝΓΘ
ΔΌZ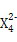 ΨΏ”–«Ω―θΜ·–‘,Ρή…±ΨζœϊΕΨ,ΜΙ‘≠≤ζΈο «Z3+ΓΘ
ΨΏ”–«Ω―θΜ·–‘,Ρή…±ΨζœϊΕΨ,ΜΙ‘≠≤ζΈο «Z3+ΓΘ
ΔΎΚ§ΗΟάκΉ”ΒΡ―Έ”ΟΉς–θΡΐΦΝΒΡ‘≠“ρ «(”ΟάκΉ”ΖΫ≥Χ ΫΚΆΦρ“ΣΒΡΈΡΉ÷ΥΒΟς) ΓΘ