��Ŀ����
����Ŀ�������Ǹ������Դ���⣬���������������������������̲��ŷḻ�Ŀ������ˮ�к��д����Ļ�ѧ���ʣ��DZ���Ļ�ѧ��Դ����ˮ������Һ�幤ҵԭ�������������ɴӺ�ˮ����ȡ������ʳ�Ρ�þ���塢�⡢�ص��������ʡ������Ǻ�ˮ�ۺ����õIJ������̣�

����������⣺
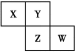
(1)����˵���������________(����)��
A����ⱥ��ʳ��ˮʱ�����ĵ缫��ӦʽΪ��Cl2��2e��===2Cl��
B����±�������һ������������ԭ��Ӧ
C���������������ᾧ
D��E���ʿ���ѭ��ʹ��
(2)������A��Դ��ʯ��Ҥ����д��A�Ļ�ѧʽ��A_______________��
(3)ʵ�����ᴿ���ε�ʵ���������Ϊ��ȡ����________�����������ˡ�________��
(4)��ҵ��������Ĺ��������У�̼�ữʱ����������Ϊ___________��������Ӧ�Ļ�ѧ����ʽ��____________����ԭ����______________��
(5)̼�ữ����ˣ���ҺD����Ҫ�ijɷ���________(��д��ѧʽ)��������һ�ɷֵ������ӵľ��巽����___________������ҺD��ͨ����������ϸСʳ�ο�������ȴ��������Ʒ��ͨ������������________(����)����������ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%���ϡ�
a������NH![]() ��Ũ�ȣ�ʹNH4Cl���������
��Ũ�ȣ�ʹNH4Cl���������
b��ʹNaHCO3���������
c��ʹNaHCO3ת��ΪNa2CO3�����������NH4Cl����
(6)�ü��ȷֽ�ķ����ⶨ��Ʒ����Ĵ��ȣ���ȡm1 g������Ʒ��ʵ�������Ƶù�������Ϊm2 g����Ĵ���Ϊ______________��
���𰸡�AC Ca(OH)2��CaO �ܽ� �����ᾧ �о�������(����ֻ���) NaCl��NH3��CO2��H2O=NH4Cl��NaHCO3�� ̼�����Ƶ��ܽ�Ƚ�С NH4Cl ȡ�����������ữ���ټ����������а�ɫ��������˵����������ΪCl�� a ![]() ��100%
��100%
��������
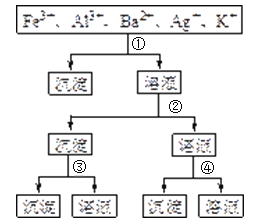
��������ͼ����ˮ������������õ���±�ʹ��Σ������к����Ȼ�þ�����ʣ���Ҫ�����������ȥ�����˺�õ���Ϊ�����ı���ʳ��ˮ����ʳ��ˮ������ͨ�백���Ͷ�����̼����̼�����ƾ�����Ȼ����Һ�����DΪ�Ȼ����Һ��̼�����Ʒֽ������̼���ƺͶ�����̼��EΪ������̼����±�к��л���̬���壬����һϵ�в��������������壬�ݴ˷������
(1)A����ⱥ��ʳ��ˮʱ�����������ӷŵ磬�缫��ӦʽΪ��2Cl��-2e��=Cl2����A����B����±�е���Ϊ����̬����������������嵥�ʣ�һ������������ԭ��Ӧ����B��ȷ��C����������ͼ���������ǹ��˳����ɵ�̼�����ƾ��壬��C����D��E����Ϊ������̼��������̼�ữ������ѭ��ʹ�ã���D��ȷ����ѡAC��
(2)��������ͼ�����������A�dz�ȥ�����е�����þ���ӵȣ��ҳ�����A��Դ��ʯ��Ҥ��������A�Ļ�ѧʽΪCa(OH)2��CaO�� �ʴ�Ϊ��Ca(OH)2��CaO��
(3)ʵ�����ᴿ���ε�������������Ϊ��ȡ�����ܽ⡢���������ˡ���������ȴ�ᾧ�����ˡ���ɼ�������Һ�������ֽ��Ե����ֽ���ˣ���ʹ��Һ��Ȼ���ǣ��ʴ�Ϊ���ܽ⣻�����ᾧ��
(4)��ҵ��������������У�̼�ữʱ���Ȼ��ơ�������ˮ�Ͷ�����̼��Ӧ����̼�����ƺ��Ȼ�泥�̼���Ƶ��ܽ�ȱ�̼�����Ƶ��ܽ�ȴ���̼�����ƾ�����������Ӧ�Ļ�ѧ����ʽΪNaCl��NH3��CO2��H2O=NH4Cl��NaHCO3�����ʴ�Ϊ���о���������NaCl��NH3��CO2��H2O=NH4Cl��NaHCO3����̼�����Ƶ��ܽ�Ƚ�С��
(5)���ݲ������̣�������ͨ�������̼����Һ�������ֽⷴӦ��H2O+CO2+NH3+NaCl=NaHCO3+NH4Cl�����յõ�NH4Cl����NaHCO3�ȣ�̼�������ܽ�Ⱥ�С���ᾧ�����ữ����Һ����Ҫ�ɷ�ΪNH4Cl���������е�������ʱ��Ҫ����ȡ�����������ữ���ټ����������а�ɫ��������ҺD����Ҫ�ijɷ���NH4Cl��ͨ��������������NH4+��Ũ�ȣ�ʹNH4Cl�������������Һ��ʣ����Ȼ��ƿ����������ã���ѡa���ʴ�Ϊ��NH4Cl��ȡ������Һ���ȼ������ữ���ٵμ���������Һ���а�ɫ������˵�����������������ӣ�a��
(6)����ǰ���������Ϊm1�����Ⱥ������Ϊm2���������ʧ������Ϊ��m1-m2�����ȹ����з����ķ�ӦΪ2NaHCO3![]() Na2CO3+H2O +CO2��
Na2CO3+H2O +CO2��
2NaHCO3![]() Na2CO3+H2O +CO2 ������
Na2CO3+H2O +CO2 ������
2��84 62
m(NaHCO3) m1-m2
���![]() =
=![]() �����m(NaHCO3)=
�����m(NaHCO3)= ![]() ������Ʒ��̼���Ƶ�����= m1-
������Ʒ��̼���Ƶ�����= m1-![]() =
=![]() ��̼���Ƶ���������=
��̼���Ƶ���������=![]() ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ��![]() ��100%��
��100%��

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д�����Ŀ����2 L���ܱ������ڣ�800��ʱ��Ӧ2NO(g)��O2(g)2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)(mol) | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��1��д���÷�Ӧ��ƽ�ⳣ������ʽ��K��___________________________����֪��K300��>K350������÷�Ӧ��______�ȷ�Ӧ��
��2����ͼ��ʾNO2�仯��������____����O2��ʾ��0��2 s�ڸ÷�Ӧ��ƽ������v��_________��

��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����________��
a��v(NO2)��2v(O2) b��������ѹǿ���ֲ���
c��v��(NO)��2v��(O2) d���������ܶȱ��ֲ���
��4��Ϊʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ�����________������������������������������������
a����ʱ�����NO2���� b���ʵ������¶�
c������O2��Ũ�� d��ѡ���Ч����
����Ŀ����Դ�������������ᷢչ�Ļ������о���ѧ��Ӧ�е������仯�������ڸ��õ����û�ѧ��ӦΪ������������ش��й����⣺
��1����һ�������£�2H2O==2H2��+O2��,��ͼ����ȷ��ʾ�÷�Ӧ�������仯����________(��A��B��ʾ)��

�Ӷϼ��ͳɼ��ĽǶȷ���������Ӧ�������ı仯����ѧ���ļ������±���������1molˮ�����仯Ϊ________kJ��
��ѧ�� | H��H | O��O | H��O |
����kJ/mol | 436 | 496 | 463 |
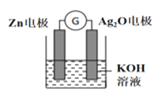
��2���������������о���������ѧ������ܵ��ת������п�����һ�ֳ�����ѧ��Դ���䷴Ӧԭ����Zn��Ag2O��H2O=Zn(OH)2��2Ag���乤��ʾ����ͼ��ʾ����װ����Ag2O��_______��(��������Һ�е�K+��_____�缫�ƶ����Zn����Ag2O������Ag2O�缫����______(��ԭ��������Ӧ�����缫��ӦʽΪ_____________________��
��3��һ���¶��£���3molA�����1molB����ͨ��һ�ݻ��̶�Ϊ1L���ܱ������У��������·�Ӧ��3A(g)��B(g)![]() xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ_______��xΪ_________;��ʱ��Ӧ��B��ת����Ϊ_________������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ��_____0.8mol/L������ڣ�С�ڻ���ڡ�����
xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ_______��xΪ_________;��ʱ��Ӧ��B��ת����Ϊ_________������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ��_____0.8mol/L������ڣ�С�ڻ���ڡ�����